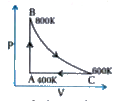
One mole of diatomic ideal gas undergoes a cyclic process ABC as shown in figure. The process BC is adiabatic. The temperature at A, B and C are 400K, 800K and 600 K respectively. Choose the correct statememt.

The change in internal energy in the process AB is
The change in internal energy in the process BC is
The change in internal energy in whole cyclic process is 250 R
The change in internal energy in the process CA is 700 R
The correct Answer is:B
△UAB=nCv(TB−TA)=1×5R2(800−400)=1000R
△UBC=nCv(TC−TB)=1×5R2(600−800)=−500R
△U total =0
△UCA=nCv(TA−TC)=1×5R2(400−600)=−500R.
Topper's Solved these Questions
- GASEOUS STATE & THERMODYNAMICSBook:VMC MODULES ENGLISHChapter:GASEOUS STATE & THERMODYNAMICSExercise:JEE ADVANCED (ARCHIVE )
Explore 109 Videos
- GASEOUS STATE & THERMODYNAMICSBook:VMC MODULES ENGLISHChapter:GASEOUS STATE & THERMODYNAMICSExercise:Level - 2
Explore 40 Videos
- ENERGY & MOMENTUMBook:VMC MODULES ENGLISHChapter:ENERGY & MOMENTUMExercise:JEE ADVANCE (ARCHIVE) - TRUE/FALSE TYPE
Explore 1 Video
- GRAVITATIONBook:VMC MODULES ENGLISHChapter:GRAVITATIONExercise:JEE Advance (Archive) TRUE/FALSE
Explore 1 Video
Similar Questions
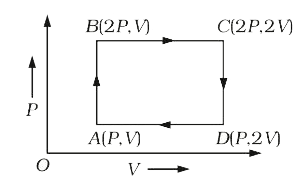
An ideal gas is taken around the cycle ABCDA as shown in the P-V diagram. What is the work done during the cycle?
View SolutionA closed vessel of fixed volume contains a mass m of an ideal gas, the root mean square speed being v. Additional mass m of the same gas is pumped into the vessel and the pressure rises to 2P, the temperature remaining the same as before. The root mean square speed of the molecules now is :
View SolutionTwo particles of mass 1 kg and 3 kg move towards each other under their mutual force of attraction. No other force acts on them. When the relative velocity of approach of the two particles is 2m/s, their centre of mass has a velocity of 0.5 m/s. When the relative velocity of approach becomes 3m/s, the velocity of the centre of mass is 0.75 m/s.
View SolutionIs it possible to put an artificial satellite in an orbit in such a way that it always remain visible directly over New Delhi ?
View SolutionOne mole of a diatomic ideal gas undergoes a cyclic process ABC as shown in figure. The process BC is adiabatic. The temperature at A,B and C are 400K, 800K and 600K respectively. Choose the correct statement:
View SolutionOne mole of an ideal monoatomic gas undergoes a cyclic process as shown in figure. Then the change in the energy in expanding the gas from a to c along the path abc is:
View SolutionOne mole of an ideal monoatomic gas undergoes a cyclic process as shown in figure . Temperature at point 1 =300K and process 2−3 is isothermal .
_S01_115_O01.png)
The efficiency of cycle is
View SolutionOne mole of an ideal monoatomic gas undergoes a cyclic process, as shown in the figure. If the temperature of the gas at state 1 is 300 K and at state 4 is 500 K, then heat exchanged during process 2→3, is
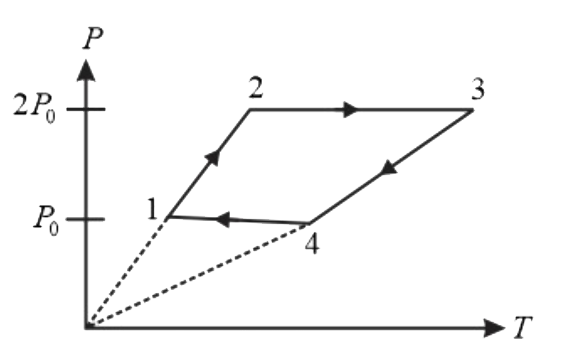
View SolutionOne mole of an ideal monoatomic gas undergoes a cyclic process, as shown in the figure. If the temperature of the gas at state 1 is 300 K and at state 4 is 500 K, then heat exchanged during process 2rarr3 , is
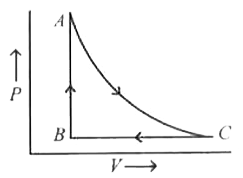
View SolutionOne mole of ideal gas undergoes a cyclic process ACBA as shown in figure. Process AC is adiabatic. The temperatures at 1, B and C are 300 K,600 K and 450 K respectively. Choose the correct statement.
View Solutionद्विपरमाणुक आदर्श गैस का एक मोल चक्रीय प्रक्रिया ABC से गुजरता है जैसा कि चित्र में दर्शाया गया है। प्रक्रिया BC रूद्धोष्ण है। A, B एवं C के तापमान क्रमशः 400K, 800K एवं 600K है। सही कथन चुनिये
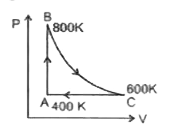
View SolutionOne mole of diatomic ideal gas undergoes a cyclic process ABC as shown in figure. The process BC is adiatomic. The temperature of A, B and C are 400 K, 800 K and 600 K respectively. Choose the correct statement ____
View SolutionOne mole of diatomic ideal gas undergoes a cyclic process ABC as shown in figure. The process BC is adiabatic. The temperature at A,B and C are 400K, 800K and 600K respectively. Choose the correct statement:
View SolutionOne mole of a diatomic ideal gas undergoes a cyclic process ABC as shown in figure. The process BC is adiabatic. The temperature at A,B and C are 400K, 800K and 600K respectively. Choose the correct statement:
View SolutionOne mole of diatomic ideal gas undergoes a cyclic process ABC as shown in figure. The process BC is adiabatic. The temperatures at A, B and C are 400K, 800K and 600K respectively.Choose the correct statement: https://haygot.s3.amazonaws.com/questions/117871.png
View SolutionTwo moles of Helium gas undergos a cyclic process as shown in figure. Assuming gas to be ideal, what is the net work involved in the cyclic process?
View Solution
