Find : (i) No.of moles in 1020 atoms of Cu
(ii) Mass of 200168O atoms in amu
(iii) Mass of 100 atoms of N in gm
(iv) No.of molecules & atoms in 54gmH2O
(v) No.of atoms in 88gmCO2 .
The correct Answer is:(i)1020NAmoles (ii)3200amu (iii)14×1.66×10−24100g (iv)3Na,9N9 (v)6NA
Step by step video solution for Find : (i) No.of moles in 10^(20) atoms of Cu (ii) Mass of 200_(8)^(16)O atoms in amu (iii) Mass of 100 atoms of N in gm (iv) No.of molecules & atoms in 54 gmH_(2)O (v) No.of atoms in 88gmCO_(2) . by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- MOLE CONCEPTBOOK - ALLENCHAPTER - MOLE CONCEPTEXERCISE - S-II
18 Videos
- MOLE CONCEPTBOOK - ALLENCHAPTER - MOLE CONCEPTEXERCISE - O-I
45 Videos
- MOLE CONCEPTBOOK - ALLENCHAPTER - MOLE CONCEPTEXERCISE - Basic Exercise
29 Videos
- METALLURGYBOOK - ALLENCHAPTER - METALLURGYEXERCISE - EXERCISE-05 [B]
26 Videos
- p-Block Element BOOK - ALLENCHAPTER - p-Block Element EXERCISE - All Questions
20 Videos
Similar Questions
Two substances P4 &O2 are allowed to react completely to form mixture of P4O6 &P4O10 leaving none of the reactants .Using this information calculate the moles of P4O6 and P4O10 in the final mixture when the following amounts of P4 & O2 are taken.
P4+3O2→P4O6
P4+5O2→P4O10
(i) If 1 mole P4 & 4 mole of O2
(ii) If 3 mole P4 & 11 mole of O2
(iii) If 3 mole P4 & 13 mole of O2
View SolutionOne atomic mass unit in kilogram is
View Solution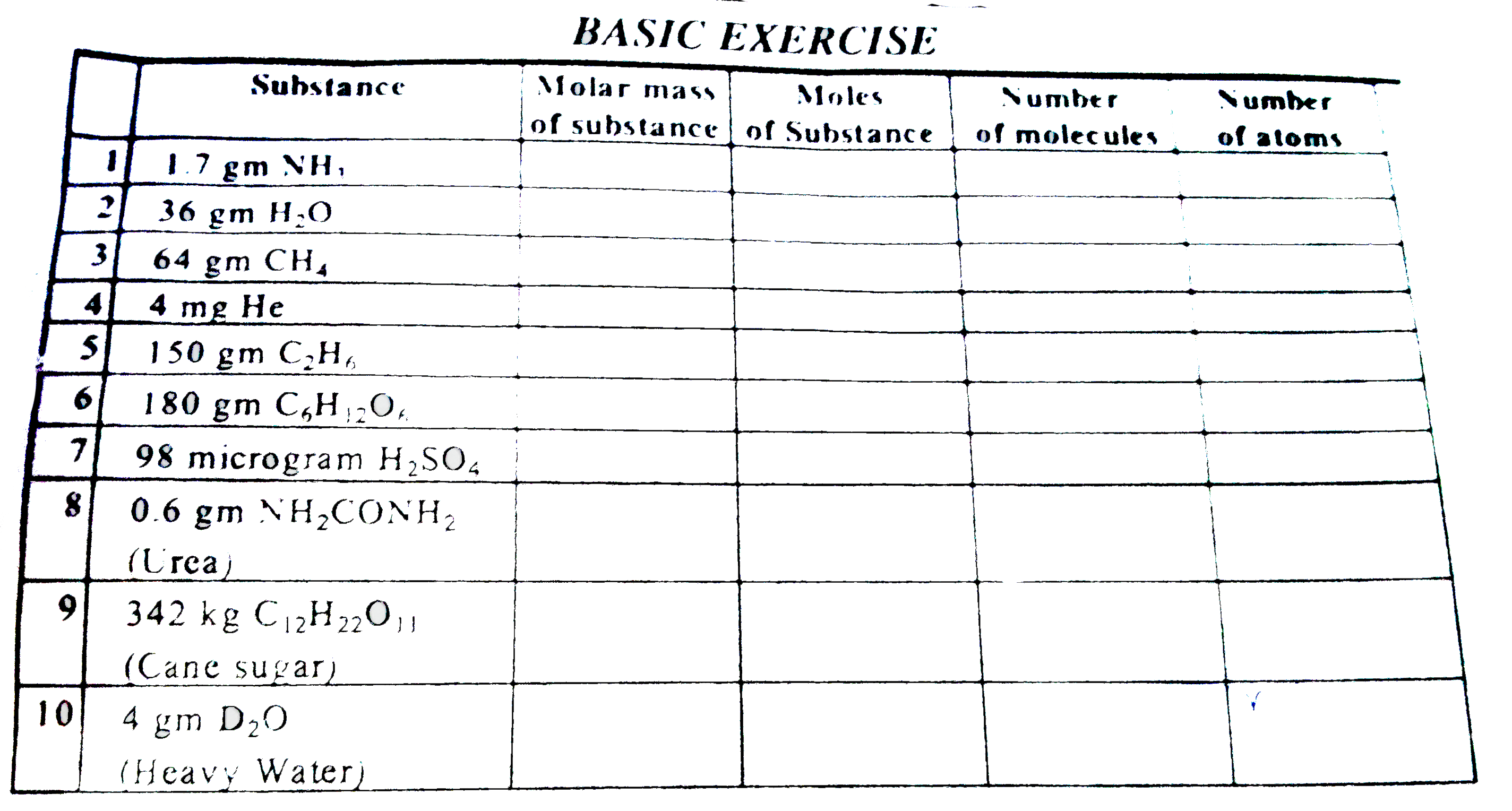
A box contains 12×1022 number of oxygen atoms. Find moles of O-atoms? (NA=6×1023)
View SolutionWhen the ore haematite is burnt in air with coke around 2000K along with lime, the process not only produces steel but also produces a silicate slag that is useful in making building materials such as cement. Discuss the same and show through balanced chemical equation.
View SolutionThe main factor responsible for weak acidic nature of BF3 is :-
View SolutionThe atomic mass of .8O16=15.9949 amu. Calculate the BE/nucleon for this atom. Mass 1n and 1p is 2.016490 amu and me=0.00055 amu.
View SolutionFind : (i) No.of moles in 1020 atoms of Cu
(ii) Mass of 200168O atoms in amu
(iii) Mass of 100 atoms of _(7).14N in gm
(iv) No.of molecules & atoms in 54gmH2O
(v) No.of atoms in 88gmCO2 .
View SolutionMaximum no.of 'O' atoms are bonded with each Si in SiO_(2) .
View SolutionThe atomic mass of an element is 50 (i) Calculate the mass of one atom, in amu (ii) Calculate the mass of 6.022xx10^(23) atoms, in gm (iii) Calculate the number of atoms in its 10 gm (iv) What mass of the element contains 3.011xx10^(20) atoms
View Solution2 moles of H_(2)SO_(4) is kept in a beaker. Find (i) Moles of H-atoms (ii) Moles of S-atoms (iii) Moles of O-atoms (iv) Number of O-atoms
View SolutionFind (I) No. of moles of cu atom In 10^(20) atoms of cu. (ii) Mass of 200 atoms in amu. (iii) Mass of 100 atoms of in amu . (iv) No. of molecules & atoms in 54 gm H_(2)O . (v) No. of atoms in 88 gm CO_(2) .
View SolutionDistinguish between the following .
(i) Atomic and molecular mass
(ii) Atomic mass and atomic weight
(iii) Empirical and molecular formula
(iv) Moles and molecules.
View SolutionAn atom has mass "no.37" and atomic number "17" .find the no.of protons (a) 21 (b) 22 (c) 17 (d) 20
View SolutionArrange the following in the order of increasing mass (atomic mass: O = 16, Cu = 63, N = 14)
I. one atom of oxygen
II. one atom of nitrogen
III. 1×10−10 mole of oxygen
IV. 1×10−10 mole of copper
View SolutionQuestions on RAM, AM and GAM|| no.OF Gm-atoms and Atoms,Introduction OF Molecule,Atomicity
View SolutionWhat is the group no.of an element having atomic no.105?
View Solution

