Among the following, the number of compounds that can react with PCl5 to give POCl3 is O2,CO2,SO2,H2O,H2SO4,P4O10.
The correct Answer is:4
Step by step video, text & image solution for Among the following, the number of compounds that can react with PCl_(5) to give POCl_(3) is O_(2),CO_(2),SO_(2),H_(2)O,H_(2)SO_(4),P_(4)O_(10). by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- JEE ADVANCEDBOOK - JEE ADVANCED PREVIOUS YEARCHAPTER - JEE ADVANCEDEXERCISE - CHEMISTRY SECTION - IV : Matric Match Type
2 Videos
- JEE ADVANCEDBOOK - JEE ADVANCED PREVIOUS YEARCHAPTER - JEE ADVANCEDEXERCISE - CHEMISTRY SECTION - II : Multiple Correct Answer Type
4 Videos
- JEE (ADVANCED ) 2020BOOK - JEE ADVANCED PREVIOUS YEARCHAPTER - JEE (ADVANCED ) 2020EXERCISE - SECTION -III
6 Videos
- JEE ADVANCED 2020BOOK - JEE ADVANCED PREVIOUS YEARCHAPTER - JEE ADVANCED 2020EXERCISE - SECTION-3
6 Videos
Similar Questions
Match the transformation in column I with appropriate options in column II

View SolutionReduction of the metal centre in aqueous permanganate ion involves
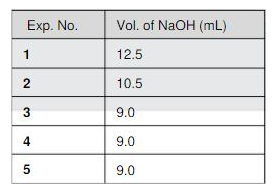
View Solution5.00 mL of 0.10 M oxalic acid solution taken in a conical flask is titrated against NaOH from a burette using phenolphthalein indicator. The volume of NaOH required for the appearance of permanent faint pink color is tabulated below for five experiments. What is the concentration, in molarity, of the NaOH solution?
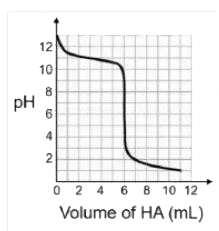
View SolutionA solution of 0 .1 weak base (B )is titrated with 0.1 M of a strong acid ( HA) . The variation of pH of the solution will be the volume of HA added is shown in the figure below. What is the pKb of the base ? The neutralization reaction is given by B+HA→HA→BH++A−
View SolutionIn the reaction, H_(2)SO_(4)+P_(2)O_(5)overset(Delta)to (X)+SO_(3) , the product (X) is :
View SolutionAmong the following, the number of compounds that can react with PCl5 to give POCl3 is O2,CO2,SO2,H2O,H2SO4,P4O10.
View SolutionN_(2)O_(4), (HPO_(2))_(2), H_(2)CO_(3), SO_(2), SO_(3), P_(4)O_(10) H_(2)SO_(4), N_(2)O_(3), HNO_(3), H_(3)PO_(3) . (a) Among the above compounds, compounds having at least one p pi - p pi bond are x. (b) Among the above compounds, compounds having at least one d pi - p pi bond are y. Given the answer as x + y
View SolutionHeavy water reacts respectively with CO_(2),SO_(3), P_(2)O_(5) and N_(2)O_(5) to give the compounds :
View SolutionAmong the following, the number of compounds that can react with PCl_(5) to give POCl_(3)" is ":O_(2),CO_(2),SO_(2),H_(2)O,H_(2)SO_(4),P_(4)O_(10) .
View SolutionH_(4)underline(S_(2))O_(6)+H_(2)O to H_(2)SO_(3)+H_(2)SO_(4)
View SolutionAmong the following, the number of compounds that can react with PCl5 to give POCl3 is : O2,CO2,SO2,H2O,H2SO4,P4O10.
View SolutionAmong the following compounds of phosphorus:
H4P2O6,P2O5,P4O6,P4O10.
The compounds having P-O-P bond is(are):
View SolutionAmong the following, the number of compounds that can react with PCl5 to give POCl3 is O2,CO2,SO2,H2O,H2SO4,P4O10.
View Solutionनिम्न में उन यौगिकों की संख्या जो PCl5 से क्रिया करके POCl3 बना सकते है
O2,CO2,SO2,H2O,H2SO4,P4O10
View Solution(vii) H_(2)C_(2)O_(4) +KMnO_(4)+H_(2)SO_(4)to H_(2)SO_(4)+MnSO_(3)+CO_(2)+H_(2)O .
View SolutionBalance the following equations by oxidation number method (i) Kr_(2)Cr_(2)O_(7) + KI + H_(2)SO_(4) to K_(2)SO_(4) + Cr_(2)(SO_(4))_(3) + I_(2) + H_(2)O (ii) KMnO_(4) + Na_(2)SO_(3) to MnO_(2) + Na_(2)SO_(4) + KOH (iii) Cu + HNO_(3) to Cu(NO_(3))_(2) + H_(2)O (iv) KMnO_(4) + H_(2)C_(2)O_(4) + H_(2)SO_(4) to K_(2)SO_(4) + MnSO_(4) + CO_(2) + H_(2)O
View Solution

