Which of the following pairs would have higher boiling points ?
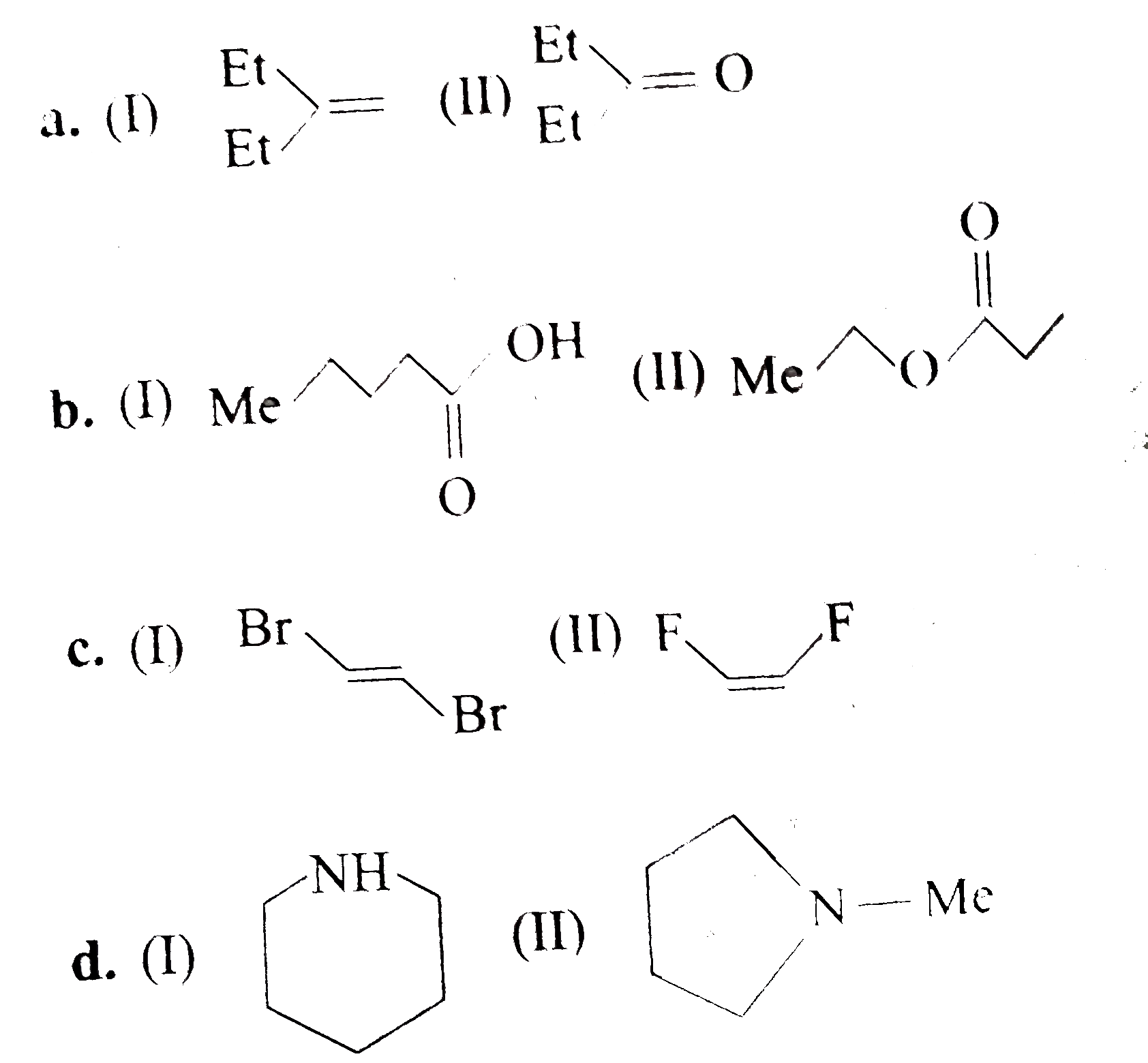
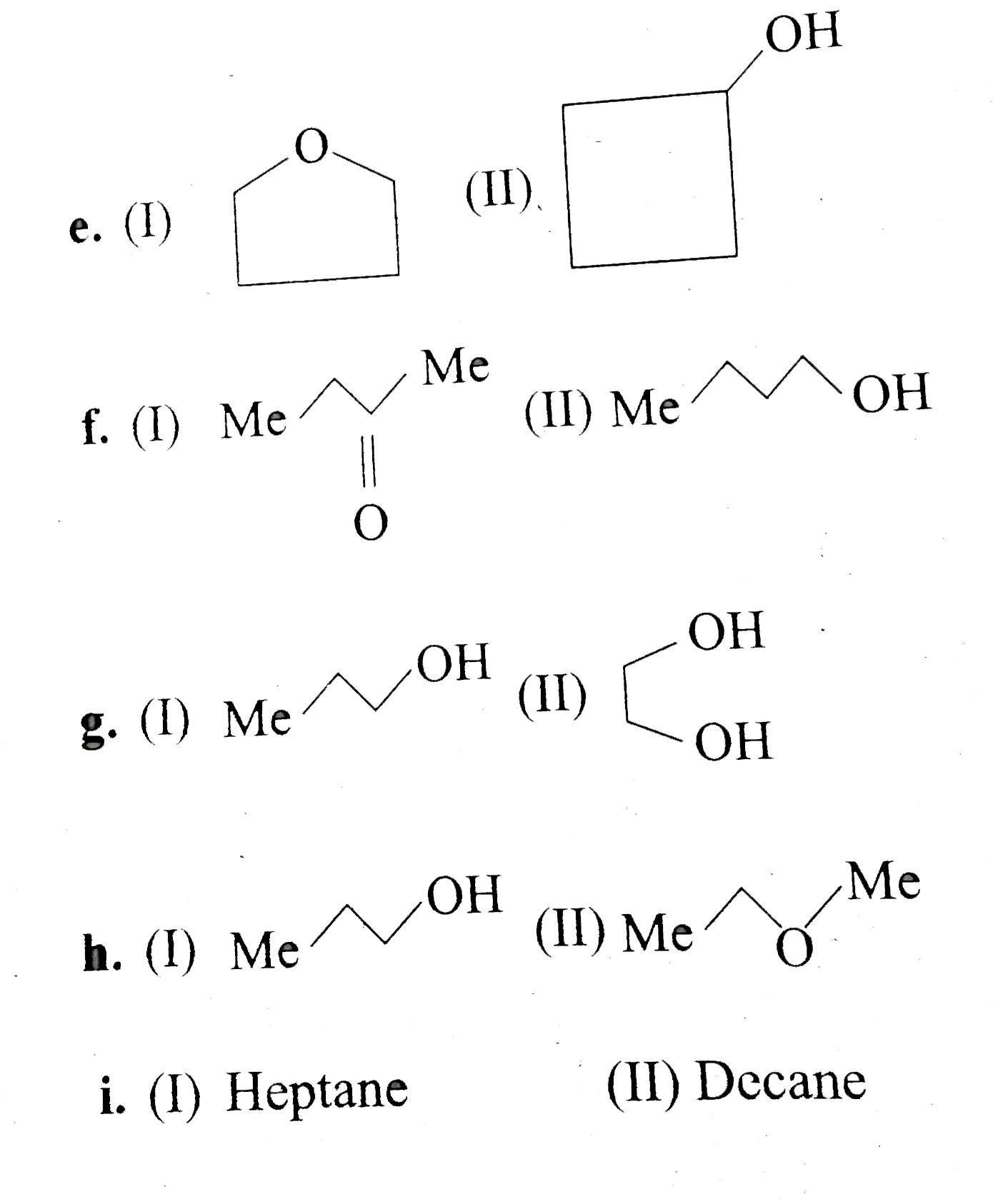
(a) (II) because carbonyl group is more polar than double bond.
(b) (I) acid forms dimar due to H−bonding.
( c) (II) cis-compound has high dipole moment (μ), so more polar than trans-compound (μ=0)
(d) (I) it forms H−bonding1 through H atom in (−NH) group.
( e) (II) it forms H−bonding
(f) (II) alcohols form H-bonding.
(g) (II) two (OH) groups can form more H−bonding with each other.
(h) (I) alcohold form H−bonding
(i) (II) because of high molecular mass and large size, have larger van der Waals forces of attraction.
Topper's Solved these Questions
- GENERAL ORGANIC CHEMISTRYBOOK - CENGAGE CHEMISTRYCHAPTER - GENERAL ORGANIC CHEMISTRYEXERCISE - Subjective
11 Videos
- GENERAL ORGANIC CHEMISTRYBOOK - CENGAGE CHEMISTRYCHAPTER - GENERAL ORGANIC CHEMISTRYEXERCISE - Concept
16 Videos
- GENERAL ORGANIC CHEMISTRYBOOK - CENGAGE CHEMISTRYCHAPTER - GENERAL ORGANIC CHEMISTRYEXERCISE - Analytical and Descriptive
1 Video
- CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDSBOOK - CENGAGE CHEMISTRYCHAPTER - CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDSEXERCISE - Analytical and Descriptive Type
3 Videos
- HYDROGEN, WATER AND HYDROGEN PEROXIDEBOOK - CENGAGE CHEMISTRYCHAPTER - HYDROGEN, WATER AND HYDROGEN PEROXIDEEXERCISE - Subjective Archive (Subjective)
3 Videos
Similar Questions
Give the decreasing order of acidic character of the following :
(a) (1) Benzene
(2) CH3OH
(3) H2O
(4) CH3SH
(b) (1) CH3CH2COOH
(2) HOCH2CH2COOH
(3) C1CH2CH2COOH
(4) O2NCH2CH2COOH
( c)
(d) (1) [Fe(H2O)6]2+
(2) [Fe(H2O)6]3+
(3) [A1(H2O)6]3+
(4) [Cr(H2O)6]3+
( e) (1) HCHO
(2) CH3CHO
PhCHO
(4) CH3−O∣∣C−CH3 (5) R−O∣∣C−CI
(6) (CH3CO)2
(7) (RCOOR)
(8) RCONH2.
View SolutionUsing the curved-arrow notation, show the formation of reactive intermediates when the following convalent bonds undergo heterolytic cleavage :
(a) CH3−S−CH3
(b) CH3−CN
( c) CH3−Cu .
View SolutionArrange the following in the increasing order of their basicities.
(I) p−Toluidine
(II) N,N−Dimethyl-p-toluidine
(III) p−Nitroaniline
(IV) Aniline.
View SolutionGive the IUPAC name of the following compound.
CH2=CH−CH(CH3)2
View SolutionH2O2 is a better oxidising agents than water.
View SolutionThe element having the higher boiling point is
View SolutionIn which of the following pairs does the first compound not have a higher boiling point than the second ?
View SolutionWhich isomer will have a higher boiling point?
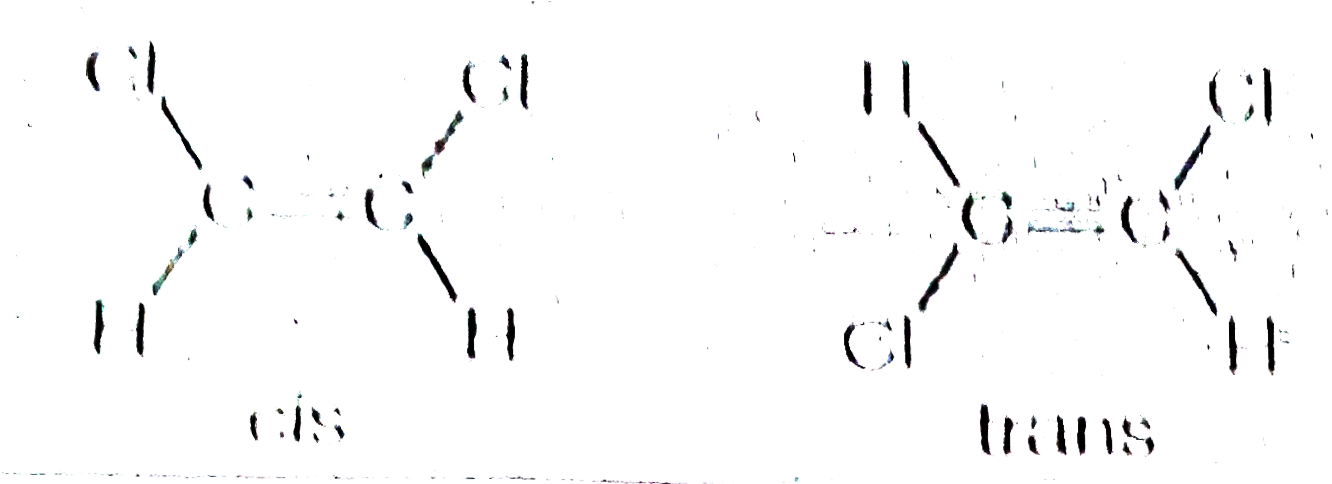
View SolutionWhich of the following equation solution will have higher boiling point at1atm. Pressure ?
View Solutionनिम्न युग्मों में किसके क्वथनांक अधिक है ? समपक्ष -विपक्ष 1 -Cholropropene
View SolutionIn which of the following pairs, the first compound does not have higher boiling point than the second ?
View SolutionWhich isomer will have higher boiling point ?
View SolutionThe boiling point of which is higher
View SolutionWhich of the following will have a higher boiling point
View SolutionWhich of the following isomeric amines is likely to have higher boiling point
View Solution

