form the following data for the reaction between A and B,

(a) Calculate the order of the reaction with respect to A and with respect to B.
(b) Calculate the rate constant at 300K.
( c) Calculate the pre-expontential factor.
Lat rate expression be r=K[A]m[B]n
(i) ∴5.0×10−4=K[2.5×10−4]m[3.0×10−5]n…(1)
4.0×10−3=K[5.0×10−4]m[6.0×10−5]n…(2)
1.6×10−2=K[1.0×10−3]m[6.0×10−5]n…(3)
By eqs. (2) and (3), m=2
By eqs. (1) and (2), n=1
∴ Rate =K[A]2[B]1
or Order with respect to A and B are 2 and 1 respectively.
(ii) By eq. (1)
5.0×10−4=K[2.5×10−4]2[3×10−5]1
∴K=2.66×108litre2mol−2sec−1 at 300K
(iii) ∵2.303log10K2K1=EaR[T2−T1]T1T2,[∵r2r1=K2K1]
∵2.303log102.0×10−35.0×10−4=Ea8.314[320−300300×320]
Ea=55.33kJ
(iv) Also 2.303log10K=2.303log10A−EaRT
2.303log102.66×108=2.303log10A−55.33×1038.314×300
∴A=1.140×1018
Similar Questions
From the following data for the reaction between A and B
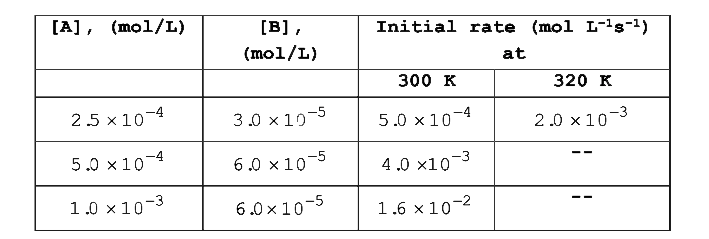
Calculate:
(i) the order of the reaction with respect to A and with respect to B
(ii) the rate constant at 300 K
(iii) the pre-exponential factor.
View SolutionFrom the following data for the reaction between A and B
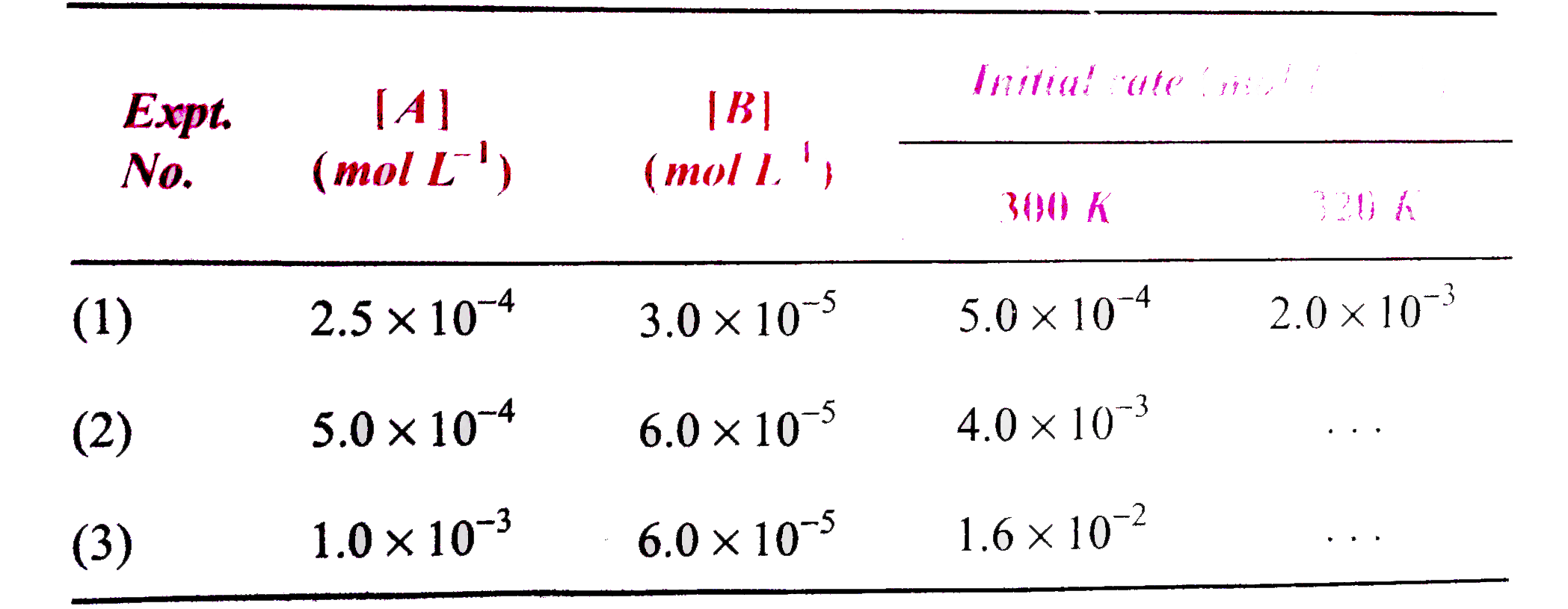
Calculate the following
(i) The order of the reaction with respect to A and with respect to B
(ii) The rate constant at 300K
(iii) The energy of activation and
(iv) The pre-exponential factor.
View Solution
Knowledge Check
if the rate of reaction between A and B is given by rate =k[A][Bn] then the reaction is
Afirst order in ABnth order in BCover all order is (1+n)Dall are correct
Consider the following data for the reaction:
A+B→Products

Determine the order of reaction with respect to A and with respect to B and the overall order of the reaction.
View SolutionCalculate the order of the reaction A→ product from the following data :
 .
.
View SolutionCalculate the order of reaction A+B→P, with respect to A from the following data :

View SolutionThe reaction between A and B is first order with respect to A and zero order with respect to B. Fill in the blanks in the following table :

View SolutionThe reaction between A and B is first order with respect to A and zero order with respect to B. Fill in the blanks in the following table:
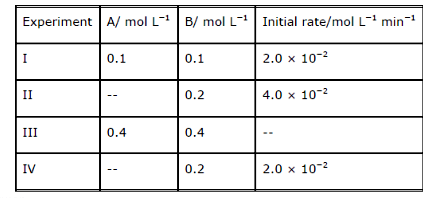
View SolutionThe reaction between A and B is first order with respect to A and zero order with respect to B. Fill in the blanks in the following table.

View Solutionform the following data for the decompoistion of N2O5 in carbon tertrachloride solution at 321K, show that the reaction is of the first order and calculate the rate constant.
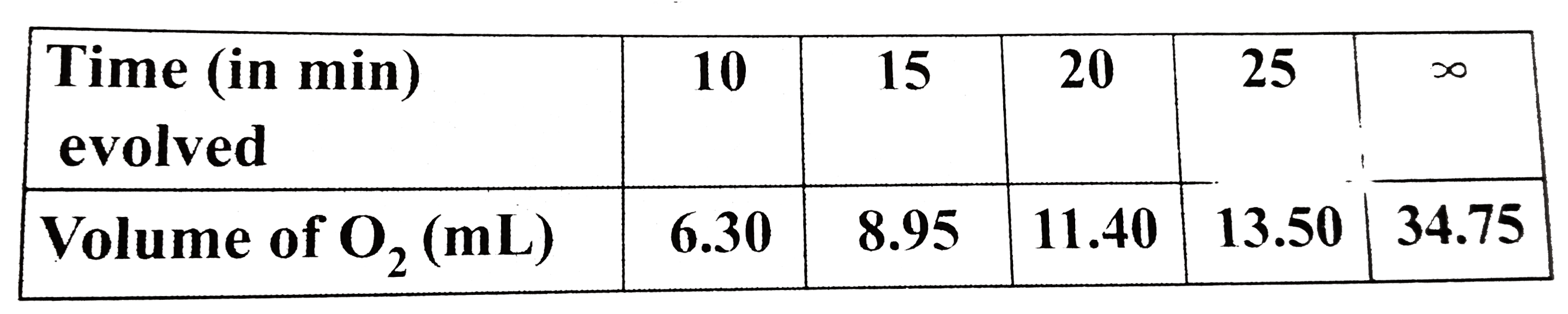
View Solution

