A gas may expand either adiabatically or isothermally. A number of p−V curves are drawn for the two processes over different ranges of pressure and volume, it will be found that
(i) Two adiabatic curves do not intersect
(ii) two isothermal curves do not intersect
(iii) an adiabatic curve and an isothermal curve may intersect.
(iv) the magnitude of the slope of an adiabatic curve is greater than the magnitude of the slope of an isothermal curve
two adiabatic curves do not intersect
two isothermal curves do not intersect
an adiabatic curve and an isothermal curve may intersect
the magnitude of the slope of an isothermal curve for the same values of pressure and volume
Step by step video & image solution for A gas may expand either adiabatically or isothermally. A number of p-V curves are drawn for the two processes over different ranges of pressure and volume, it will be found that (i) Two adiabatic curves do not intersect (ii) two isothermal curves do not intersect (iii) an adiabatic curve and an isothermal curve may intersect. (iv) the magnitude of the slope of an adiabatic curve is greater than the magnitude of the slope of an isothermal curve by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
A small , hollow metal cylinder is closed at one end . Its mass is known . Which of the following instruments are required to find the density of the metal ?
View SolutionSome physical quanties are given in Column I and some possible SI units in which these quantities may be expressed are given in Column II. Match the physical quantities in Column I with the units in Column II.
Column IColumn II(A)GMeMs(p)(volt)(coulomb)(metre)G−universal gravitational constantMe−mass of the earth,Ms−mass of the sun(B)3RTM(q)(kilogram)(metre)3(second)−2R−universal gas constant,T−absolute temperature, M- molar mass(C)F2q2B2(r)(metre)2(second)−2F−force,q−charge,B−magnetic field(D)GMeRe(s)(farad)(volt)2(kg)−1G−universal gravitational constant,Me−mass of the earth,Re−radius of the earth.
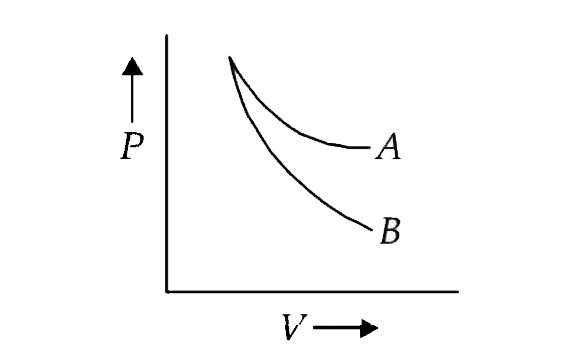
View SolutionThe curves A and B in the figure shown P-V graphs for an isothermal and an adiabatic process for an idea gas. The isothermal process is represented by the curve A.
View SolutionA gas may expand either adiabatically or isothermally. A number of p−V curves are drawn for the two processes over different ranges of pressure and volume, it will be found that
(i) Two adiabatic curves do not intersect
(ii) two isothermal curves do not intersect
(iii) an adiabatic curve and an isothermal curve may intersect.
(iv) the magnitude of the slope of an adiabatic curve is greater thanthe magnitude of the slope of an isothermal curve
View SolutionThe ratio of slopes of adiabatic and isothermal curves is
View SolutionA gas may expand either abiabatically or isothermally .A number of P-V curves are drawn for the two processes over different ranges of pressure and volume . It will be found that :
View SolutionA gas may expand either abiabatically or isothermally .A number of P-V curves are drawn for the two processes over different ranges of pressure and volume . It will be found that :
View SolutionThe slope of adiabatic curve is _________________than the slope of an isothermal curve.
View SolutionThe slope of isothermal and adiabatic curves are related as
View SolutionThe relation between the slope of isothermal curve and slope of adiabatic curve is
View SolutionThe slope of adiabatic P-V curve is_____times the slope of isothermal P-V curve in the process.
View SolutionA gas may expands either adiabatically or isothermally. A number of p-V curves are drawn for the two process over different ranges of pressure and volume. It will be found that a) Two adiabatic curves do not intersect b) Two isothermal curves do not intersect c) An adiabatic curve and an isothermal curve may intersect d) The magnitude of the slope of an adiabatic curve is greater than the magnitude of the slope of an isothermal curve for the same values of pressure and volume
View SolutionA gas may expands adiabatically or Isothermally. If P,V graphs are drawn and if s_(1) and s_(2) are the slope of the process then a) s_(1)gts_(2) b) adiabatic curve and Isothermal curve may intersect c) s_(1)lts_(2)
View SolutionA gas may expand either adiabatically or isothermally. A number of P-V curves are drawn for the two processes over different range of pressure and volume. It will be found that
View SolutionA gas may expand either adiabatically or isothermally. A number of p-V curves are drawn for the two process over different ranges of pressure and volume. It will be found that a) Two adiabatic curves do not intersect b) Two isothermal curves do not intersect c) An adiabatic curve and an isothermal curve may intersect d) The magnitude of the slope of an adiabatic curve is greater than the magnitude of the slope of an isothermal curve for the same values of pressure and volume
View SolutionThe curves A and B in the figure shown P-V graphs for an isothermal and an adiabatic process for an idea gas. The isothermal process is represented by the curve A.
View Solution
