The noble gases are chemically inert due to high ionization enthalpy, positive electron gains enthalpy and presence of completely filled orbitals. However, Xe forms a number of compounds such as XePtF6XeF2,XeF4,XeF6 and a number of xenon oxides and oxy fluorides. Xenon fluorides react with fluoride ion acceptors such as PF5,AsF5,SbF3 etc. to form cationic species but with fluoride ion donors such as alkali metal fluorides they forms anionic fluoroanions. They are also hydrolysed by water but their reactivity increases with oxidation state of Xe.
The shape and hybridisation of some Xenon oxygen fluorides are given. Select the wrong answer.
XeOF2 - T shaped - sp3d
XeOF4 - Square pyramidal - sp3d2
XeO2F2 - Trigonal bipyramidal - sp3d
XeO3F2 - Tetrahedral - sp3.
The correct Answer is:D
Step by step video, text & image solution for The noble gases are chemically inert due to high ionization enthalpy, positive electron gains enthalpy and presence of completely filled orbitals. However, Xe forms a number of compounds such as XePtF_(6) XeF_(2), XeF_(4), XeF_(6) and a number of xenon oxides and oxy fluorides. Xenon fluorides react with fluoride ion acceptors such as PF_(5), AsF_(5), SbF_(3) etc. to form cationic species but with fluoride ion donors such as alkali metal fluorides they forms anionic fluoroanions. They are also hydrolysed by water but their reactivity increases with oxidation state of Xe. The shape and hybridisation of some Xenon oxygen fluorides are given. Select the wrong answer. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- THE NOBLE GASESBook:DINESH PUBLICATIONChapter:THE NOBLE GASESExercise:Matrix Match Type
Explore 5 Videos
- THE NOBLE GASESBook:DINESH PUBLICATIONChapter:THE NOBLE GASESExercise:Assertion Reason
Explore 10 Videos
- THE NOBLE GASESBook:DINESH PUBLICATIONChapter:THE NOBLE GASESExercise:RQ
Explore 96 Videos
- THE NITROGEN FAMILYBook:DINESH PUBLICATIONChapter:THE NITROGEN FAMILYExercise:Ultimate
Explore 14 Videos
- THE OXYGEN FAMILYBook:DINESH PUBLICATIONChapter:THE OXYGEN FAMILYExercise:PREPARATORY PACKAGE
Explore 14 Videos
Similar Questions
Here each question contains statements given in two column which have to be matched. Statements in columns I are labelled as A, B, C and D where as the statements in column II are labelled p, q, r and s. The answers to these questions are to be appropriately bubbled as illustrated in the following example.
If the correct matches should like the following.
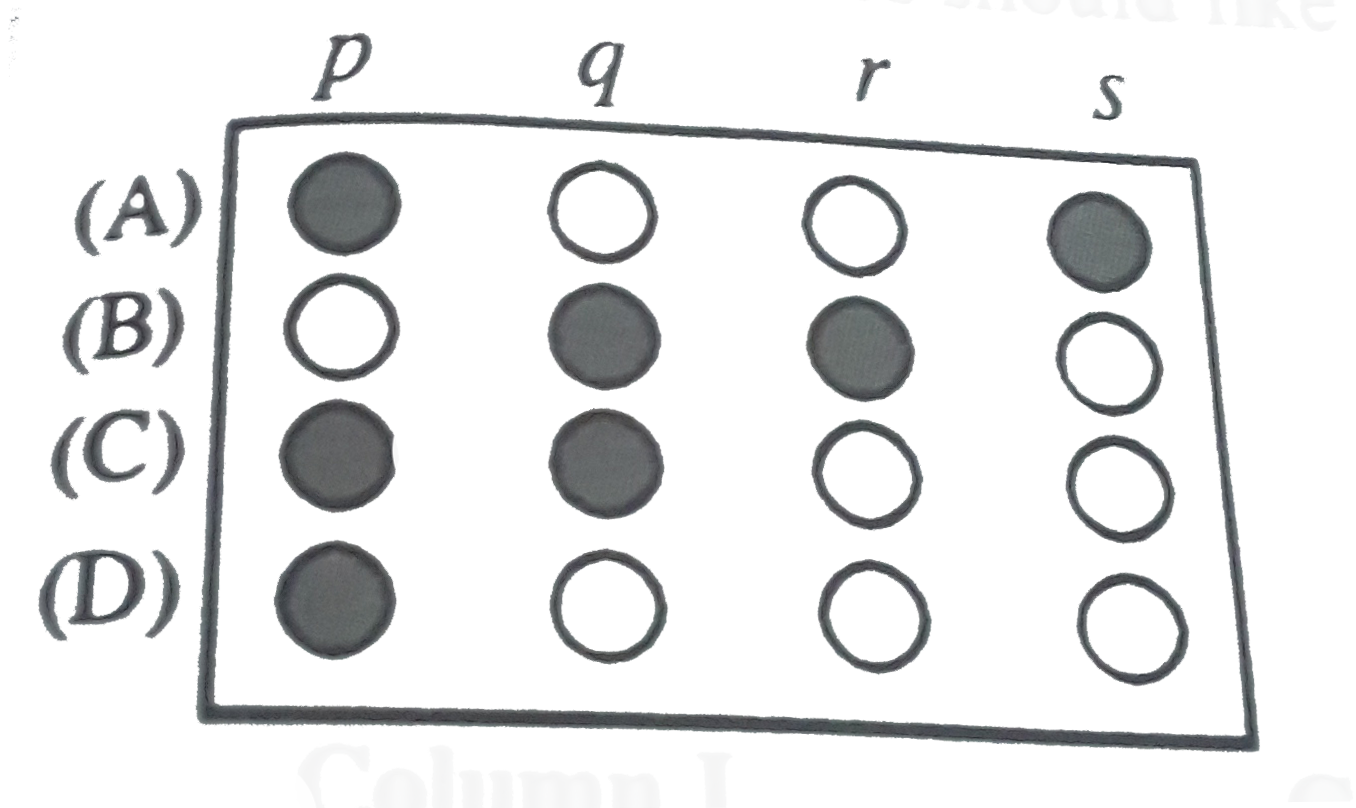
Column IColumn II(A)NH3(p)Basic(B)PH3(q)sp3 hybridized(C)H2S(r)Acidic(D)H2O(s)Hydrogen bonded
View SolutionAssertion :Solubility of noble gases in water decreases with increases in atomic size
Reason :Solubility of noble gases in water is due to instantaneous dipole induced dipole interaction
View SolutionThe last orbit of argon would have electrons
View SolutionOrdinary phosphine is spontaneously inflammable due to the presence of
View SolutionThe element which shows maximum catenation in Group 16 is
View SolutionS1: Argon is used in arc welding of metals or alloys to provide an inert atomosphere.
S2:XeF2,XeF4 and XeF6 are colourless crystalline solids and sublime readily at 298K.
S3:XeF2,XeF4 and XeF6 are readily hydrolysed.
S4: Xenon fluorides react with flourine ion acceptor to form cationic species and fluoride ion donors to form fluoro anions.
View SolutionHow are xenon fluorides XeF2, XeF4 and XeF6 obtained?
View SolutionXenon does not form fluorides such as XeF3 and XeF5. Explain.
View SolutionThe noble gases are chemically inert due to high ionization enthalpy, positive electron gains enthalpy and presence of completely filled orbitals. However, Xe forms a number of compounds such as XePtF6XeF2,XeF4,XeF6 and a number of xenon oxides and oxy fluorides. Xenon fluorides react with fluoride ion acceptors such as PF5,AsF5,SbF3 etc. to form cationic species but with fluoride ion donors such as alkali metal fluorides they forms anionic fluoroanions. They are also hydrolysed by water but their reactivity increases with oxidation state of Xe.
The number of lone pairs and bond pairs of electrons around Xe in XeOF4 respectively are
View SolutionThe noble gases are chemically inert due to high ionization enthalpy, positive electron gains enthalpy and presence of completely filled orbitals. However, Xe forms a number of compounds such as XePtF_(6) XeF_(2), XeF_(4), XeF_(6) and a number of xenon oxides and oxy fluorides. Xenon fluorides react with fluoride ion acceptors such as PF_(5), AsF_(5), SbF_(3) etc. to form cationic species but with fluoride ion donors such as alkali metal fluorides they forms anionic fluoroanions. They are also hydrolysed by water but their reactivity increases with oxidation state of Xe. Which of the following is not formed by Xe ?
View SolutionThe noble gases are chemically inert due to high ionization enthalpy, positive electron gains enthalpy and presence of completely filled orbitals. However, Xe forms a number of compounds such as XePtF6XeF2,XeF4,XeF6 and a number of xenon oxides and oxy fluorides. Xenon fluorides react with fluoride ion acceptors such as PF5,AsF5,SbF3 etc. to form cationic species but with fluoride ion donors such as alkali metal fluorides they forms anionic fluoroanions. They are also hydrolysed by water but their reactivity increases with oxidation state of Xe.
When XeF4 is treated with water, it forms ?
View SolutionHow are xenon fluorides XeF2,XeF4 and XeF6 obtained?
View SolutionCesium fluoride reacts with XeF_(6) to form ________
View SolutionHow are Xenon fluorides XeF_(2),XeF_(4) and XeF_(6) obtained ?
View Solutionजीनॉन फ्लुओराइड, XeF2,XeF4 तथा XeF6 कैसे बनाये जाते हैं?
View Solution
