The noble gases have closed-shell electronic cordigaration and are monatomic gases under normal condition .The low bolling points of the ligher noble gases aree due to the weak dispersion points of the ligher noble gases an due to the weak dispersion forces between the atoms and the alsence of other interalumic interactions.
The direct reaction of xenon with flarine loads to a series of compounds with water oxidation number +2,−4and+6,XeF4 reactsviolenatly with water to give XeO2 .The compound of deduced axbibt nci strouchemistry and their goometries can be deduced considering the total number of electron puirs in the valence shell.
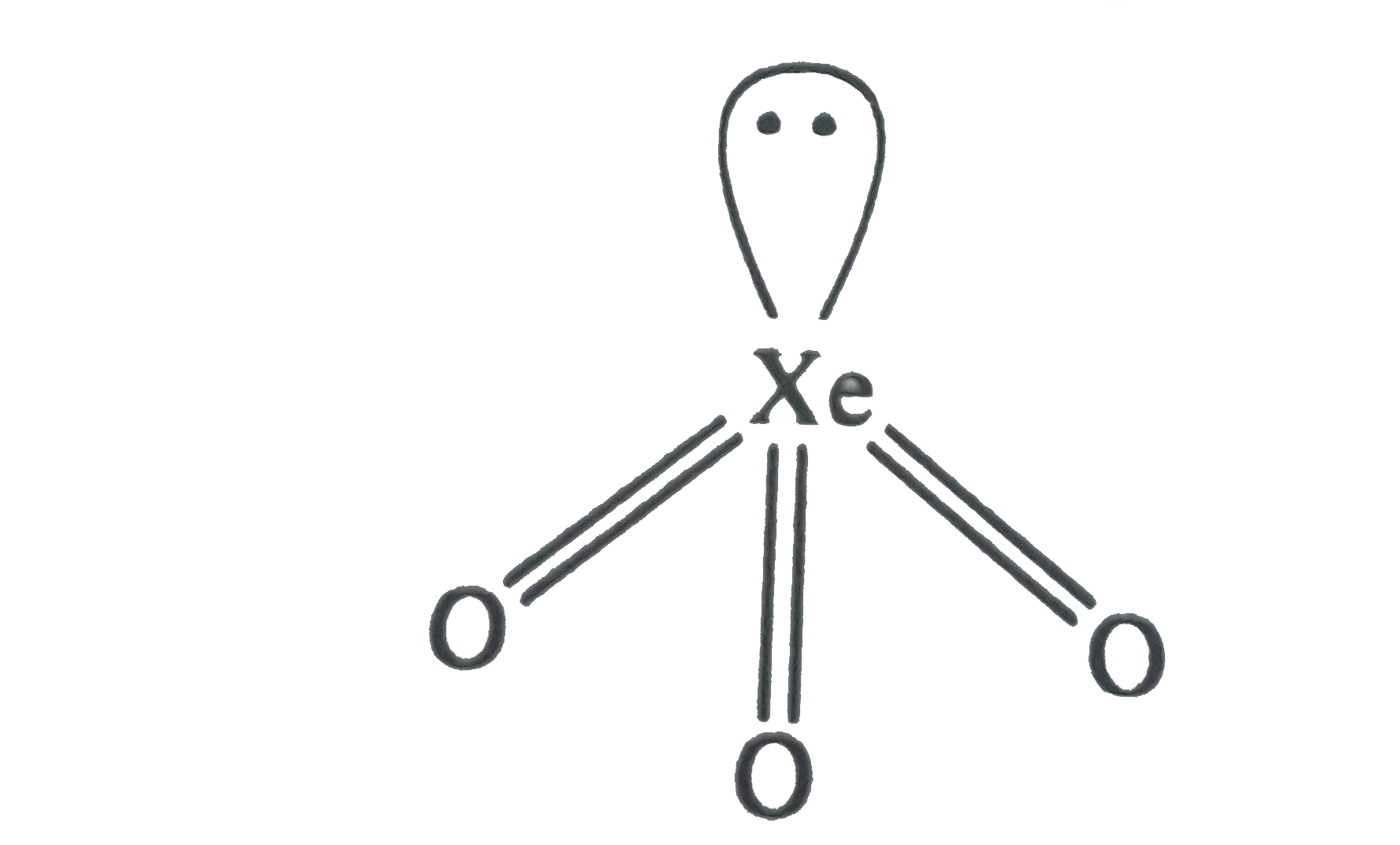
The structure of XeO3 is
linear
planar
Pyramidal
T shaped.
The correct Answer is:C
XeO3 total e− pairs = 4(3bp + lp) sp3 hybridization, pyramidal shape
Topper's Solved these Questions
- THE NOBLE GASESBOOK - DINESH PUBLICATIONCHAPTER - THE NOBLE GASESEXERCISE - Matrix Match Type
5 Videos
- THE NOBLE GASESBOOK - DINESH PUBLICATIONCHAPTER - THE NOBLE GASESEXERCISE - Assertion Reason
10 Videos
- THE NOBLE GASESBOOK - DINESH PUBLICATIONCHAPTER - THE NOBLE GASESEXERCISE - RQ
96 Videos
- THE NITROGEN FAMILYBOOK - DINESH PUBLICATIONCHAPTER - THE NITROGEN FAMILYEXERCISE - Ultimate
14 Videos
- THE OXYGEN FAMILYBOOK - DINESH PUBLICATIONCHAPTER - THE OXYGEN FAMILYEXERCISE - PREPARATORY PACKAGE
14 Videos
Similar Questions
Here each question contains statements given in two column which have to be matched. Statements in columns I are labelled as A, B, C and D where as the statements in column II are labelled p, q, r and s. The answers to these questions are to be appropriately bubbled as illustrated in the following example.
If the correct matches should like the following.
Column IColumn II(A)NH3(p)Basic(B)PH3(q)sp3 hybridized(C)H2S(r)Acidic(D)H2O(s)Hydrogen bonded
View SolutionAssertion :Solubility of noble gases in water decreases with increases in atomic size
Reason :Solubility of noble gases in water is due to instantaneous dipole induced dipole interaction
View SolutionThe last orbit of argon would have electrons
View SolutionOrdinary phosphine is spontaneously inflammable due to the presence of
View SolutionThe element which shows maximum catenation in Group 16 is
View SolutionThe noble gases have closed-shell electronic cordigaration and are monatomic gases under normal condition .The low bolling points of the ligher noble gases aree due to the weak dispersion points of the ligher noble gases an due to the weak dispersion forces between the atoms and the alsence of other interalumic interactions.
The direct reaction of xenon with flarine loads to a series of compounds with water oxidation number +2,−4and+6,XeF4 reactsviolenatly with water to give XeO2 .The compound of deduced axbibt nci strouchemistry and their goometries can be deduced considering the total number of electron puirs in the valence shell.
Argon is used in arc welding because of its
View SolutionThe noble gases have closed-shell electronic cordigaration and are monatomic gases under normal condition .The low bolling points of the ligher noble gases aree due to the weak dispersion points of the ligher noble gases an due to the weak dispersion forces between the atoms and the alsence of other interalumic interactions. The direct reaction of xenon with flarine loads to a series of compounds with water oxidation number +2, - 4 and +6, XeF_(4) reactsviolenatly with water to give XeO_(2) .The compound of deduced axbibt nci strouchemistry and their goometries can be deduced considering the total number of electron puirs in the valence shell. The structure of XeO_(3) is
View SolutionThe noble gases have closed- shell electronic configuration and are monoatomic gases under normal conditions. The low boiling points of the lighter noble gases are due to weak dispersion forces between the atoms and the absence of other interatomic interactions. The direct reaction of xenon with fluorine leads to a series of compounds with oxidation number +2,+4 and +6. XeF_(4) reacts violently with water to give XeO_(3) . The compound of xenon exhibit rich sterochemistry and their geometries can be deduced considring the total number of electron pairs in the valence shell. Argon is used in arc welding because of its:
View SolutionThe noble gases have close shell electronic configuration and are monoatomic gases under normal conditions. The low boiling points of the lighter noble gases are due to weak dispersion forces between the atoms and the absence of other inter atomic interactions. The direct reactions of xenon with fluorine leads to a series of compounds with oxidation number +2, +4 " and" +6. XeF_(4) reacts violently with water to give XeO_(3) . The compounds of xenon exhibit stereochemistry and their geometries can be deduced considering the total number of electrons in valence shell Argon is used in are welding because of its.
View SolutionThe noble gases have close shell electronic configuration and are monoatomic gases under normal conditions. The low boiling points of the lighter noble gases are due to weak dispersion forces between the atoms and the absence of other inter atomic interactions. The direct reactions of xenon with fluorine leads to a series of compounds with oxidation number +2,+4 and+6.XeF4 reacts violently with water to giveXeO3. The compounds of xenon exhibit stereochemistry and their geometries can be deduced considering the total number of electrons in valence shell
XeF4 and XeF6 are expected to be :
View SolutionStatement-1 :Noble gases have very low boiling points.
Statement-2 : Noble gases being monoatomic have weak dispersion interatomic forces.
View SolutionThe noble gases have closed-shell electronic configuration and are monoatomic gases under normal conditions. The low boiling points of the lighter noble gases are due to weak dispersion forces between the atoms and the absence of other Interatomic Interactions. The direct reaction of xenon with fluorine leads to a series of compounds with oxidation numbers +2,+4 and +6. XeF_4 reacts violently with water to give XeO_3 The compound of xenon exhibit rich stereochemistry and their geometries can be deduced considering the total number of electron pairs in the valence shell. Argon is used In arc welding because of its:
View SolutionThe noble gases have closed-shell electronic configuration and are monoatomic gases under normal conditions. The low boiling points of the lighter noble gases are due to weak dispersion forces between the atoms and the absence of other Interatomic Interactions. The direct reaction of xenon with fluorine leads to a series of compounds with oxidation numbers +2,+4 and +6. XeF_4 reacts violently with water to give XeO_3 The compound of xenon exhibit rich stereochemistry and their geometries can be deduced considering the total number of electron pairs in the valence shell. XeF_4 and XeF_6 are expected to be:
View SolutionThe noble gases have closed -shell electronic configuration and are monoatomic gases under normal conditions. The low bp of the ligher noble bp of the lighter noble gases are due to weak dispersion force between the atoms and the absence of other interatomic interactions. The direct reaction of xenon with fluorine leads to a series of compounds with oxidation numbers +2,+4 and +6 . XeF_(4) reacts violently with water to give XeO_(3) . the compound of xenone exhibit rich stereochemistry and their geometries can be deduced considering the total number of electron pairs in the valence shell. The structures of XeO_(3) is
View SolutionThe noble gases have closed -shell electronic configuration and are monoatomic gases under normal conditions. The low bp of the ligher noble bp of the lighter noble gases are due to weak dispersion force between the atoms and the absence of other interatomic interactions. The direct reaction of xenon with fluorine leads to a series of compounds with oxidation numbers +2,+4 and +6 . XeF_(4) reacts violently with water to give XeO_(3) . the compound of xenone exhibit rich stereochemistry and their geometries can be deduced considering the total number of electron pairs in the valence shell. XeF_(4) and XeF_(6) are expected to be
View SolutionAssertion (A) : Noble gases have very low boiling points.
Reason (R) : Noble gases being monoatomic have no intermolecular forces except weak dispersion forces.
View Solution

