What are the products formed when an aqueous solution of magnesium bicarbonate is boiled ?
MgCO3,H2O,CO2
Mg(HCO3)2,H2O
Mg(OH)2,H2O
Mg,CO,H2O
The correct Answer is:A
To determine the products formed when an aqueous solution of magnesium bicarbonate is boiled, we can follow these steps:
1. Identify the Chemical Formula:
The chemical formula for magnesium bicarbonate is Mg(HCO3)2.
2. Understand the Reaction Upon Boiling:
When magnesium bicarbonate is heated (or boiled), it undergoes thermal decomposition. The bicarbonate ions decompose to form carbonates, carbon dioxide, and water.
3. Write the Decomposition Reaction:
The decomposition reaction can be represented as:
Mg(HCO3)2(aq)→MgCO3(s)+CO2(g)+H2O(l)
Here, MgCO3 (magnesium carbonate) is formed as a solid precipitate, CO2 (carbon dioxide) is released as a gas, and H2O (water) remains in the solution.
4. Identify the Products:
From the reaction, we can identify the products formed:
- Magnesium carbonate (MgCO3)
- Carbon dioxide (CO2)
- Water (H2O)
5. Conclusion:
Therefore, when an aqueous solution of magnesium bicarbonate is boiled, the products formed are magnesium carbonate, carbon dioxide, and water.
Final Answer:
The products formed when an aqueous solution of magnesium bicarbonate is boiled are:
- Magnesium carbonate (MgCO3)
- Carbon dioxide (CO2)
- Water (H2O)
The chemical formula for magnesium bicarbonate is Mg(HCO3)2.
When magnesium bicarbonate is heated (or boiled), it undergoes thermal decomposition. The bicarbonate ions decompose to form carbonates, carbon dioxide, and water.
The decomposition reaction can be represented as:
Mg(HCO3)2(aq)→MgCO3(s)+CO2(g)+H2O(l)
Here, MgCO3 (magnesium carbonate) is formed as a solid precipitate, CO2 (carbon dioxide) is released as a gas, and H2O (water) remains in the solution.
From the reaction, we can identify the products formed:
- Magnesium carbonate (MgCO3)
- Carbon dioxide (CO2)
- Water (H2O)
Therefore, when an aqueous solution of magnesium bicarbonate is boiled, the products formed are magnesium carbonate, carbon dioxide, and water.
The products formed when an aqueous solution of magnesium bicarbonate is boiled are:
- Magnesium carbonate (MgCO3)
- Carbon dioxide (CO2)
- Water (H2O)
Topper's Solved these Questions
- QUALITATIVE ANALYSIS (ANION)Book:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSIS (ANION)Exercise:Match The Column
Explore 1 Video
- QUALITATIVE ANALYSIS (ANION)Book:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSIS (ANION)Exercise:Single And Double Value Integer Type
Explore 3 Videos
- QUALITATIVE ANALYSIS (ANION)Book:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSIS (ANION)Exercise:Part-II : National Standard Examination IN
Explore 8 Videos
- QUALITATIVE ANALYSISBook:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSISExercise:INORGANIC CHMISTRY(Qualitative analysis)
Explore 35 Videos
- QUALITATIVE ANALYSIS PART 1Book:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSIS PART 1Exercise:A.L.P
Explore 37 Videos
Similar Questions
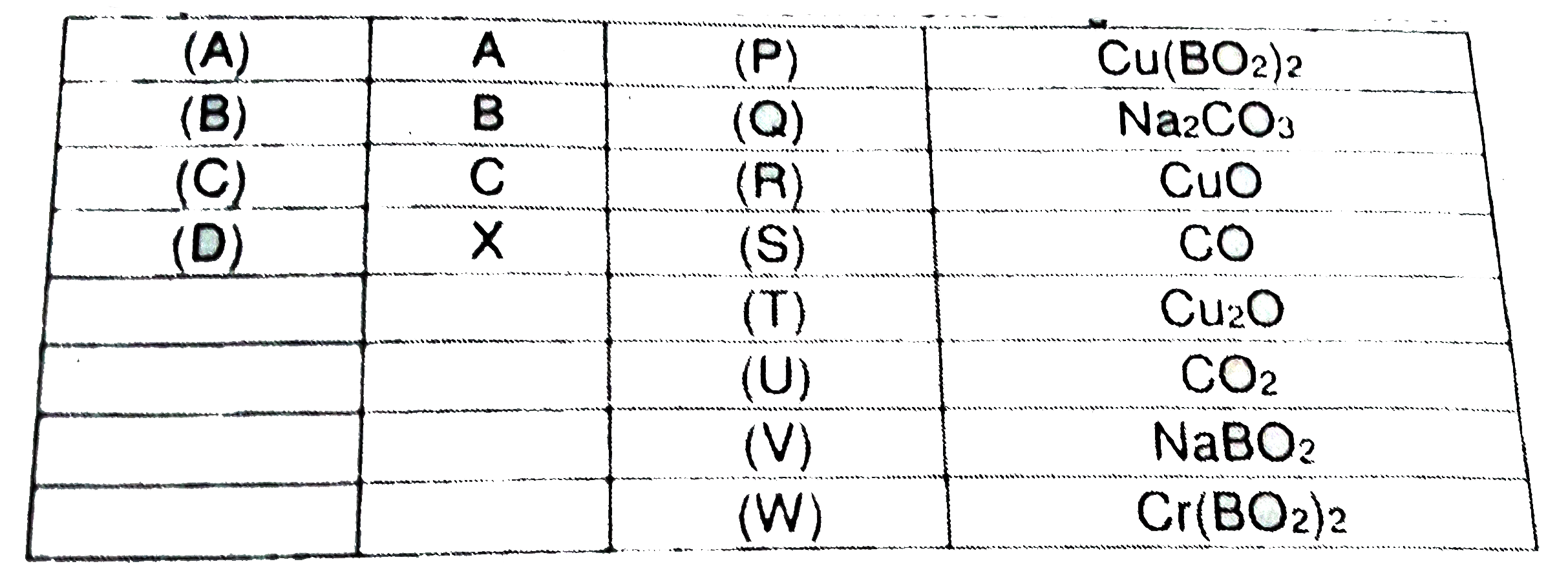
CuCO3 was strongly heated to obtain a residue A and gas B. The residue obtained was treated with a salt of sodium 'X' and oxide Y, which produced a blue colored glassy compound C on heating in oxidising flame. The same combination of x and Y gave a green colored glassy compound D when Cr2(SO4)3 was heated with them in oxidizing flame. Match the following accordingly :
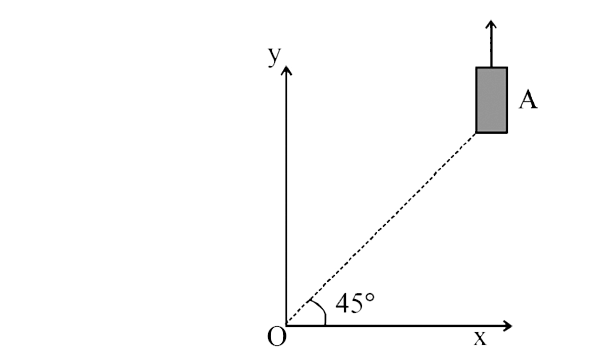
View SolutionOn a frictionless horizontal surface , assumed to be the x−y plane , a small trolley A is moving along a straight line parallel to the y−axis ( see figure) with a constant velocity of (√3−1)m/s . At a particular instant , when the line OA makes an angle of 45(∘) with the x− axis , a ball is thrown along the surface from the origin O. Its velocity makes an angle ϕ with the x− axis and it hits the trolley .
(a) The motion of the ball is observed from the frame of the trolley . Calculate the angle θ made by the velocity vector of the ball with the x−axis in this frame .
(b) Find the speed of the ball with respect to the surface , if ϕ=(4θ)/(3).
View SolutionWhich of the metal chloride is insoluble in cold water but dissolves in hot water ?
View SolutionS1: Silver iodide is fairly soluble in hypo solution.
S2: Heavy metal chloride like AgCl,HgCl2 etc. also respond to Chromyl chloride test.
S3: Bromine reacts with KI liberating violet vapours of iodine.
S4: Diphenylamine reagent test is also given by nitrites chlorates, bromates, iodates, etc. in addition to NO−3
View SolutionA red solide is insoluble in water. However it becomes soluble if some KI is added to water. On heating the red solid in a test tube, there is liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The red solid is :
View SolutionWhat products are formed when precipitate formed by the reaction of Hg2+2 ions and excess of sodium hydroxide solution is boiled?
View SolutionWhat are the products formed when an aqueous solution of magnesium bicarbonate is boiled ?
View SolutionWhen aqueous solution of benzene diazonium chloride is boiled, the product formed is
View SolutionWhen aqueous solution of benzene diazonium chloride is boiled the product formed is
View SolutionWhat are the products formed, when an aqueous solution of magnesium bicarbonate is boiled?
View SolutionWhat are the products formed when chlorine is passed through aqueous hypo solution?
View SolutionWhat are the products formed when chlorine is passed through aqueous hypo solution?
View Solutionक्या होता हैं जब बेंजल क्लोराइड को जलीय NaOH विलयन के साथ उबालते हैं?
View Solutionक्या होता है जब
एथिल एथेनोएट को जलीय KOH घोल के साथ उबाला जाता है?
View SolutionWhen aqueous solution of benzene diazonium chloride is boiled the product formed is
View Solution
