An aqueous solution of salt containing an acidic radical X-reacts with sodium hypochlorite in neutral medium. The gas evolved produces blue black colour spot on the starch paper.The anion X− is:
CH3COO−
Br−
l−
NO−2s
The correct Answer is:C
Ocl−+3l−+H2O→l−3+2OH−+Cl−
l−3+starch→ blue -black spot on starch paper appears due to the formation of iodine-starch adsorption complex.
Topper's Solved these Questions
- QUALITATIVE ANALYSIS (ANION)BOOK - RESONANCE ENGLISHCHAPTER - QUALITATIVE ANALYSIS (ANION)EXERCISE - Match The Column
1 Video
- QUALITATIVE ANALYSIS (ANION)BOOK - RESONANCE ENGLISHCHAPTER - QUALITATIVE ANALYSIS (ANION)EXERCISE - Single And Double Value Integer Type
3 Videos
- QUALITATIVE ANALYSIS (ANION)BOOK - RESONANCE ENGLISHCHAPTER - QUALITATIVE ANALYSIS (ANION)EXERCISE - Part-II : National Standard Examination IN
8 Videos
- QUALITATIVE ANALYSISBOOK - RESONANCE ENGLISHCHAPTER - QUALITATIVE ANALYSISEXERCISE - INORGANIC CHMISTRY(Qualitative analysis)
35 Videos
- QUALITATIVE ANALYSIS PART 1BOOK - RESONANCE ENGLISHCHAPTER - QUALITATIVE ANALYSIS PART 1EXERCISE - A.L.P
37 Videos
Similar Questions
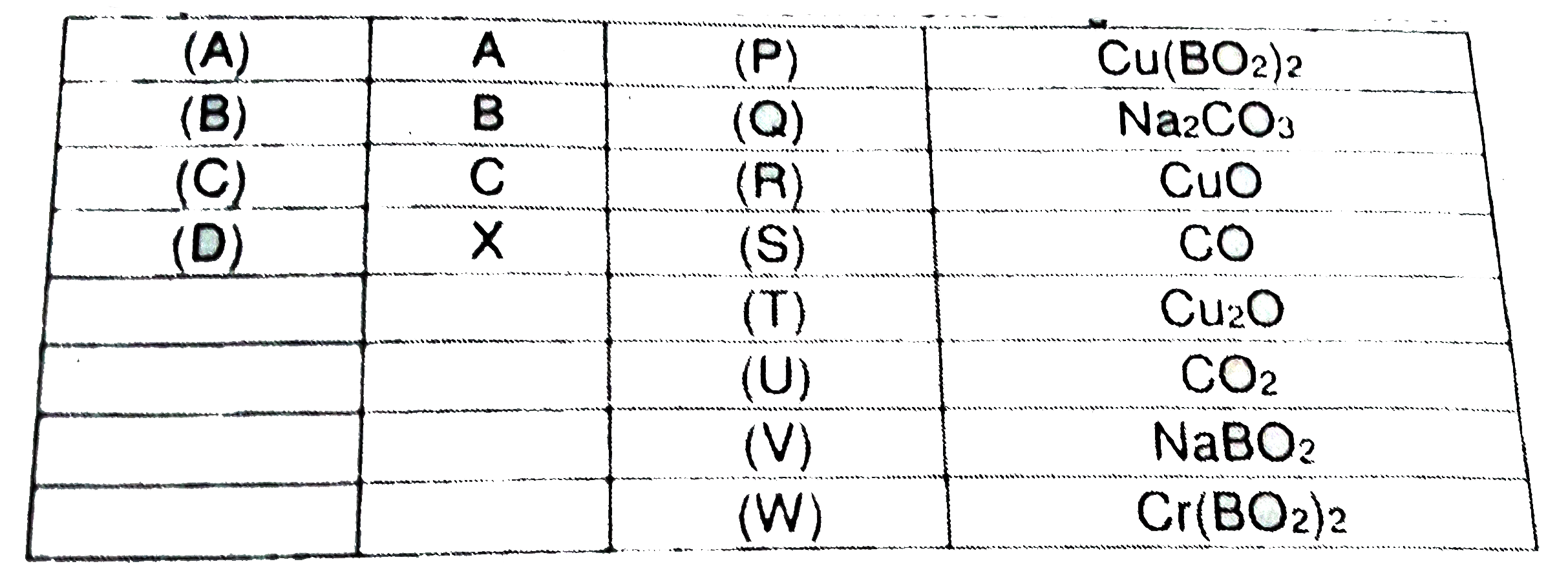
CuCO3 was strongly heated to obtain a residue A and gas B. The residue obtained was treated with a salt of sodium 'X' and oxide Y, which produced a blue colored glassy compound C on heating in oxidising flame. The same combination of x and Y gave a green colored glassy compound D when Cr2(SO4)3 was heated with them in oxidizing flame. Match the following accordingly :
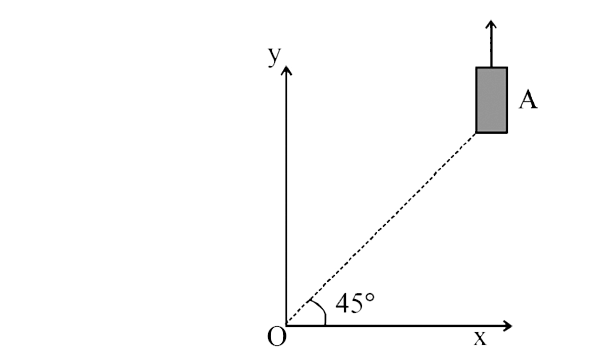
View SolutionOn a frictionless horizontal surface , assumed to be the x−y plane , a small trolley A is moving along a straight line parallel to the y−axis ( see figure) with a constant velocity of (√3−1)m/s . At a particular instant , when the line OA makes an angle of 45(∘) with the x− axis , a ball is thrown along the surface from the origin O. Its velocity makes an angle ϕ with the x− axis and it hits the trolley .
(a) The motion of the ball is observed from the frame of the trolley . Calculate the angle θ made by the velocity vector of the ball with the x−axis in this frame .
(b) Find the speed of the ball with respect to the surface , if ϕ=(4θ)/(3).
View SolutionWhich of the metal chloride is insoluble in cold water but dissolves in hot water ?
View SolutionS1: Silver iodide is fairly soluble in hypo solution.
S2: Heavy metal chloride like AgCl,HgCl2 etc. also respond to Chromyl chloride test.
S3: Bromine reacts with KI liberating violet vapours of iodine.
S4: Diphenylamine reagent test is also given by nitrites chlorates, bromates, iodates, etc. in addition to NO−3
View SolutionA red solide is insoluble in water. However it becomes soluble if some KI is added to water. On heating the red solid in a test tube, there is liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The red solid is :
View SolutionSolution of a salt in dil H2SO4 produces deep blue colour with starch iodide solution .The salt contains
View SolutionA solution of a sodium salt gives yellow precipitate with both Pb^(2+) and Ag^(+) ions. Moreover, the acidified solution of sodium salt with KNO_(2) liberates a coloured gas which turns starch paper blue. The anion is :
View SolutionThe acidic solution of a salt produced a deep blue colour with starch iodine solution. The salt may be
View SolutionA solution of a salt in concentrated sulphuric acid H2SO4 acid produced a deep blue colour with starch iodide solution.The salt may be
View SolutionAn aqueous solution of salt containing an acidic radical X -reacts with sodium hypochlorite in neutral medium. The gas evolved produces blue black colour spot on the starch paper.The anion X^(-) is:
View SolutionSolution of a salt in dilute H_(2)SO_(4) or acetic acid produces deep blue colour with starch iodide solution.The salt contains:
View SolutionSolution of a salt in dilute H2SO4 or acetic and produces deep blue colour with starch iodide solution. The salt contains :
View SolutionAn aqueous solution of salt containing an acidic radical X^(-) reacts with sodium hypochlorite in neutral medium. The gas evolved produces blue black colour spot on the starch paper. The anion X^(-) is :
View SolutionSolution of a salt in dilute H_2 SO_4 produces deep blue colour with starch iodine solution. The salt contains
View Solution(i)When silver nitrate solution is added to a salt solution of (A), a black precipitate is - obtained. (A) decolourizes acidified permanganate solution. Identify the acid radical in (A) (ii) On heating the salt, decripitation takes place. On heating a mixture of salt, manganese dioxide and conc. H_2SO_4 , a greenish yellow gas is evolved. The gas evolved turned moist blue litmus red and then turned it colourless. On performing the flame test, the salt imparted a crimson red colour to the flame. Identify the cation and anion in the salt.
View SolutionAcidic solution of a salt produced deep blue colour with starch and potassium iodide is :
View Solution

