Which of the following will not react with each other when heated together ?
BeO+MgO
Li2CO3+BeO
MgCO3+Al2O3
None of these
The correct Answer is:D
To determine which of the given compounds will not react with each other when heated together, we need to analyze each pair of compounds. Let's go through the options step by step.
1. Identify the Compounds:
- The compounds given are:
- BeO (Beryllium Oxide)
- MgO (Magnesium Oxide)
- Li2CO3 (Lithium Carbonate)
- MgCO3 (Magnesium Carbonate)
- Al2O3 (Aluminum Oxide)
- NaNO3 (Sodium Nitrate)
2. Analyze the Reactions:
- BeO + MgO:
- When heated together, BeO and MgO react to form MgBeO2.
- Conclusion: They react.
- Li2CO3 + BeO:
- When heated, Li2CO3 reacts with BeO to form Li2BeO2 and releases CO2 gas.
- Conclusion: They react.
- MgCO3 + Al2O3:
- When heated, MgCO3 reacts with Al2O3 to produce MgAl2O4 and releases CO2 gas.
- Conclusion: They react.
- NaNO3:
- Sodium Nitrate (NaNO3) generally does not react with the other compounds listed when heated. It can decompose but does not form a compound with the oxides mentioned.
- Conclusion: It does not react with the other compounds.
3. Final Conclusion:
- Among the given options, the pair that does not react when heated together is NaNO3 with any of the other compounds listed. Therefore, the correct answer is:
- D: None of these (since all the other pairs react).
- The compounds given are:
- BeO (Beryllium Oxide)
- MgO (Magnesium Oxide)
- Li2CO3 (Lithium Carbonate)
- MgCO3 (Magnesium Carbonate)
- Al2O3 (Aluminum Oxide)
- NaNO3 (Sodium Nitrate)
- BeO + MgO:
- When heated together, BeO and MgO react to form MgBeO2.
- Conclusion: They react.
- When heated, Li2CO3 reacts with BeO to form Li2BeO2 and releases CO2 gas.
- Conclusion: They react.
- When heated, MgCO3 reacts with Al2O3 to produce MgAl2O4 and releases CO2 gas.
- Conclusion: They react.
- Sodium Nitrate (NaNO3) generally does not react with the other compounds listed when heated. It can decompose but does not form a compound with the oxides mentioned.
- Conclusion: It does not react with the other compounds.
- Among the given options, the pair that does not react when heated together is NaNO3 with any of the other compounds listed. Therefore, the correct answer is:
- D: None of these (since all the other pairs react).
Topper's Solved these Questions
- QUALITATIVE ANALYSIS (ANION)Book:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSIS (ANION)Exercise:Match The Column
Explore 1 Video
- QUALITATIVE ANALYSIS (ANION)Book:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSIS (ANION)Exercise:Single And Double Value Integer Type
Explore 3 Videos
- QUALITATIVE ANALYSIS (ANION)Book:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSIS (ANION)Exercise:Part-II : National Standard Examination IN
Explore 8 Videos
- QUALITATIVE ANALYSISBook:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSISExercise:INORGANIC CHMISTRY(Qualitative analysis)
Explore 35 Videos
- QUALITATIVE ANALYSIS PART 1Book:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSIS PART 1Exercise:A.L.P
Explore 37 Videos
Similar Questions
Knowledge Check
Which of the following is not resonating structure of each other?
ACH3−N=C=SandCH3−S−C≡NBCH3−+C=OandCH3−C≡+OCCH3−O∣∣C−OHandCH3−O−∣C=+O−HDCH2=CH−C≡Nand+CH2−CH=C=N−Which of the following would be decarboxylates readily when heated?
A B
B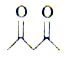 C
C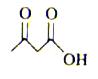 D
D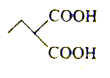
Which of the following will release CO2 when heated to 1000∘C?
View SolutionWhich of the following chloride does not react with PCl5 on heating?
View SolutionWhich of the following chloride does not react with PCl5 on heating?
View SolutionGive a specific scientific term for each of the following
Point at which two sister chromatids are held together
View SolutionWhich of the following pair(s) contain species, which react with each other on mixing their aquouos solutions to give yellow precipitate?
View SolutionWhich of the following are not functional isomers of each other ?
View Solution
