Figure displays the plot of the compression factor Z versus P for a few gases which of the following statements is/are correct for a van der Waals' gas?
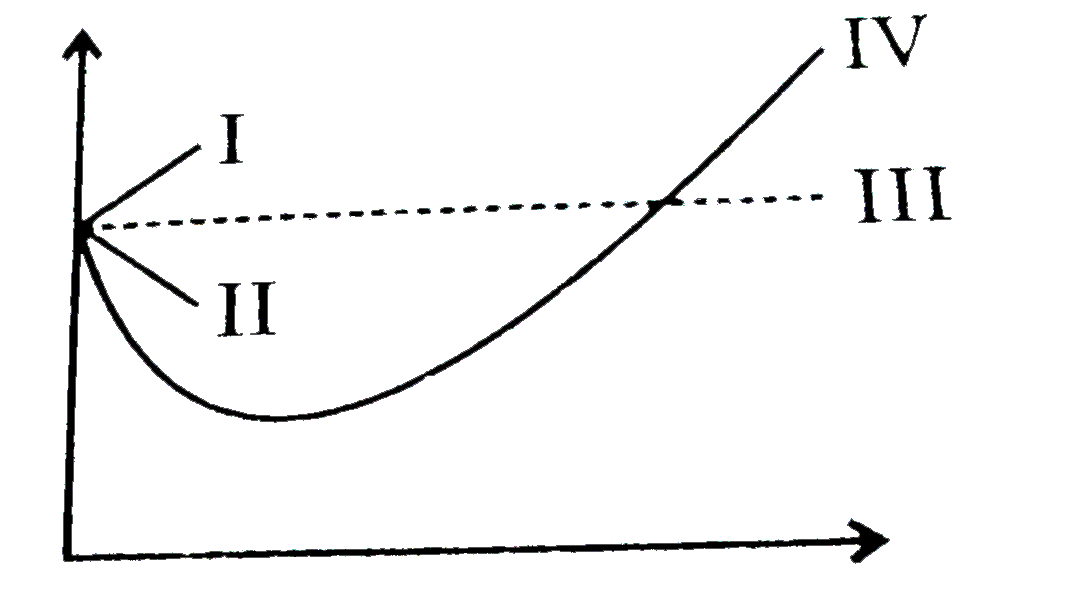
The plot I is applicable provided the van der Waals' constant a is negligible
The plot II is applicable provided the van der waals' constant b is negligible
The plot III is applicable provided the van der waals' constant a and b are negligible
the plot IV is applicable provided the temperature of theh gas is must higher than its critical temperature.
The correct Answer is:A, B, C
Topper's Solved these Questions
- GRAPHICAL INTERPRETATIONBOOK - GRB PUBLICATIONCHAPTER - GRAPHICAL INTERPRETATIONEXERCISE - Comprehension 1
1 Video
- GRAPHICAL INTERPRETATIONBOOK - GRB PUBLICATIONCHAPTER - GRAPHICAL INTERPRETATIONEXERCISE - Comprehension
49 Videos
- GRAPHICAL INTERPRETATIONBOOK - GRB PUBLICATIONCHAPTER - GRAPHICAL INTERPRETATIONEXERCISE - Electrochemistry
9 Videos
- F-BLOCK ELEMENTSBOOK - GRB PUBLICATIONCHAPTER - F-BLOCK ELEMENTSEXERCISE - Subjective type
7 Videos
- HYDROCARBON (ALIPHATIC)BOOK - GRB PUBLICATIONCHAPTER - HYDROCARBON (ALIPHATIC)EXERCISE - Previous years jee questions
28 Videos
Similar Questions
Knowledge Check
Figure displays the plot of the compression factor Z veres p for a few gases

Which of the following statements is//are correct for a van-der waals gas:AThe plot I is applicable provided the vander waals constant a is negligible.BThe plot II is a applicable provided the vander waals constant b is negligible.CThe plot III is applicable provided the vander waals constants a and b are negligible.DThe plot IV is applicable provided the temperature of the gas is much higher than its critical temperatureFigure displays the plot of the compression factor Z verses pfor a few gases.
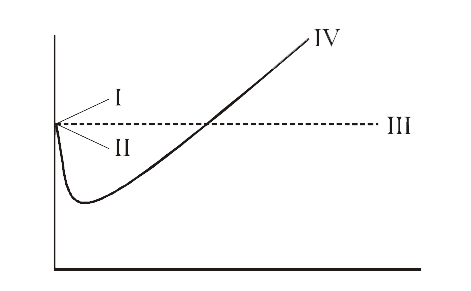
Which of the following statements is / are correct for a van-dar waals gas :AThe plot I is applicable provided the vander waals constant a is negligible.BThe plot II is applicable provided the vander waals constant b is negligible.CThe plot III is applicable provided the vander waals constants a and b are negligible.DThe plot IV is applicable provided the temperature of the gas is much higher than its critical temperature.Which is correct in case of van der Waals adsorption?
AHigh temperature, High pressureBHigh temperature, Low pressureCLow temperature, Moderate pressureDLow temperature, Low pressure
Which of the following represents the units of van der Waal constant a ?
View SolutionSelect the correct statement with reference to van der Waal's ga.
View SolutionRegarding the van der Waals constant which of the following is/are correct?
View SolutionWhich of the following noble gases has the largest van der Waal's redius?
View SolutionWhich of the following factors are responsible for origination of van der Waals forces?
View Solution

