Calculate magnitude of work involved in the reversible cyclic process ABCD if a diatomic ideal gas is involved.
![Calculate magnitude of work involved in the reversible cyclic process ABCD if a diatomic ideal gas is involved. [Given 2^(5/7) = 1.6, ln 2 = 0.7] Express answers in bar-lit and round off to next integer.](https://d10lpgp6xz60nq.cloudfront.net/physics_images/GRB_PHY_CHM_P2_V03_QB_C13_E01_415_Q01.png)
[Given 257=1.6,ln2=0.7]
Express answers in bar-lit and round off to next integer.
The correct Answer is:3
Topper's Solved these Questions
- GRAPHICAL INTERPRETATIONBook:GRB PUBLICATIONChapter:GRAPHICAL INTERPRETATIONExercise:Match The Column Type
Explore 15 Videos
- F-BLOCK ELEMENTSBook:GRB PUBLICATIONChapter:F-BLOCK ELEMENTSExercise:Subjective type
Explore 7 Videos
- HYDROCARBON (ALIPHATIC)Book:GRB PUBLICATIONChapter:HYDROCARBON (ALIPHATIC)Exercise:Previous years jee questions
Explore 28 Videos
Similar Questions
Calculate the magnitude of work done (in atm lit.) for the given cyclic process involing 1 mole of an
ideal gas .[Given : R =0.08 atm lit./mole /k]

View SolutionCalculate magnitude of net work in following cyclic process for 1 mole of an ideal monoatomic gas (in cal).
(Given: B→C isothermal process: D→A adiabatic process : TA=300K,TD=75,TD=75K,R=2cal/mole−K:ln2=0.7)
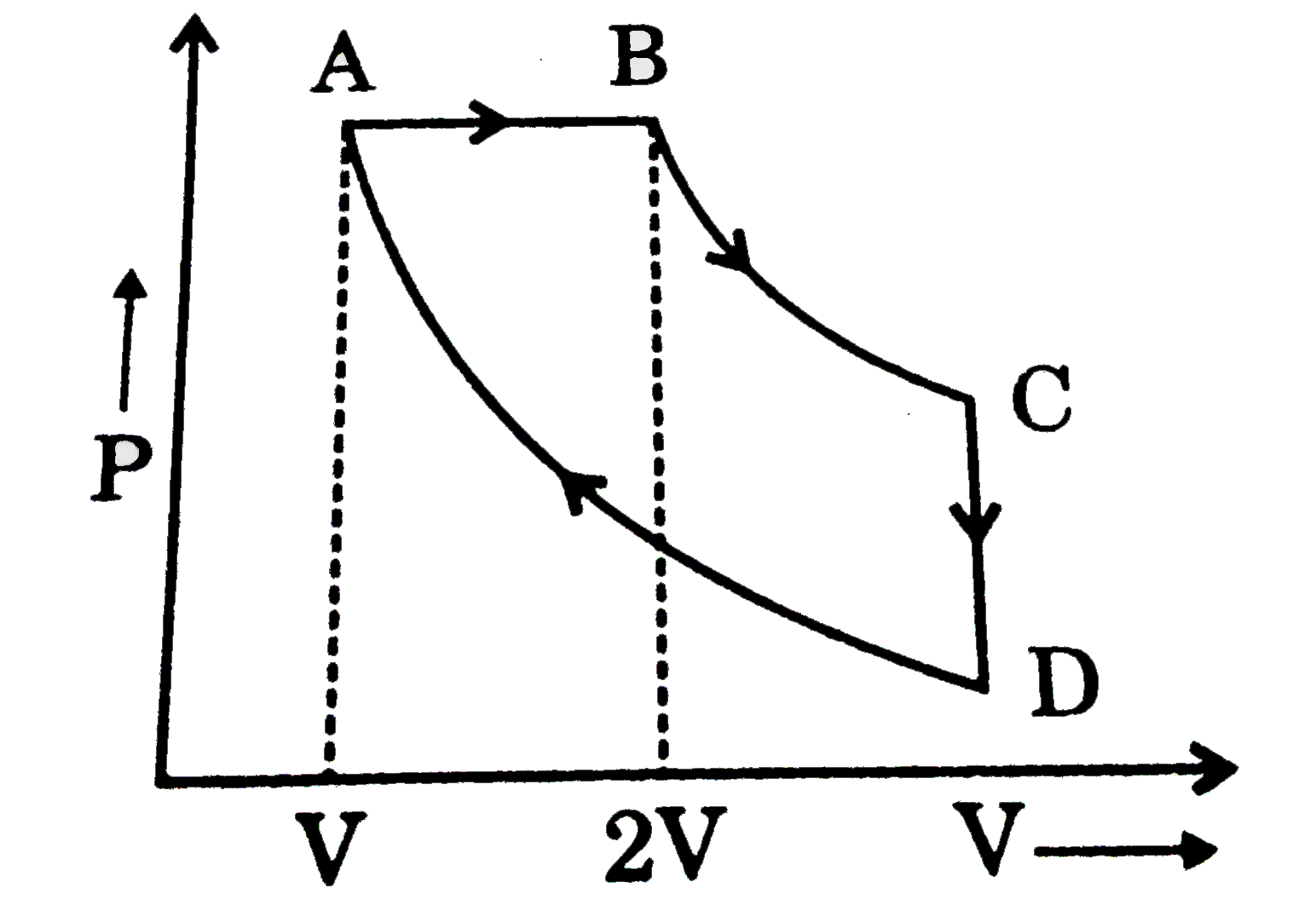
View Solution
Knowledge Check
Calculate magnitude of work done in calorie for one mole of an ideal gas subjected to the process as shown in figure. [Use: R=2cal/mol−K and ln 2=0.7]
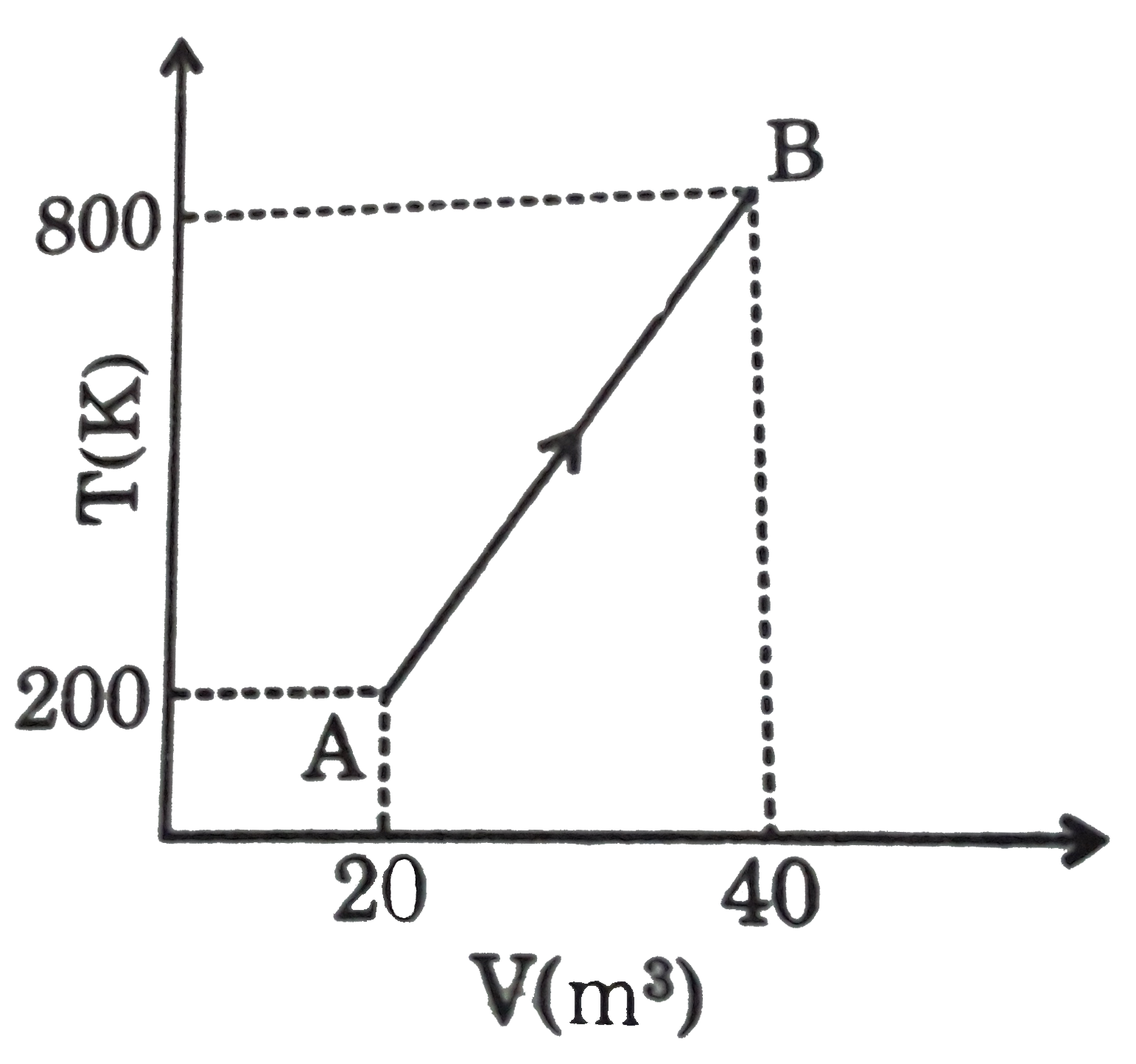 A640 calB600 calC40 calD200 cal
A640 calB600 calC40 calD200 calStaetement -1: The magniyude of the work involed in an isothermal expansion is greater than that involved in an adiabatic expansion.
Statement -2: P-v cure (pon y -axas and V on X-axas) decrease more repidly for reversible asiabatic expansion compared to reversible isothermal expansion starting from same initial state.AStatement-1 is true, Statement-2, is true, Statement -2 is a correct explanation for statement-1BStatement-1 is true, Statement-2, is true, Statement -2 is not a correct explanation for statement-1CStatement-1 is true, Statement-2 is falseDStatement-1 is false, Statement-2 is trueCalculate magnitude of work involved when 100g of calcite form of CaCO3 is converted into it's aragonite form of CaCO3 at 100 bar pressure. Given density of calcite=2g/ density of arogonite =2.5g/
A0.2kJB0kJC0.1kJD0.5kJ
Calculate total number of SO2 molecules in a sample having 32 milligrams of the SO2 gas, 1.4×1020 number of SO2 molecules, 0.8 ml of SO2 gas at 6 atm and 300 K.
[Given: NA=6×1023, R=0.08atmL/molK]
[Express your answer in terms of multiple of 1020 and then round off to nearest integer for e.g. if your answer is 6.2×1020 fill 6 in OMR after rounding off.]
View SolutionOne mole of an ideal monoatomic gas undergoes the following cyclic process. Calculate the magnitude of work (in cal) in a cycle. (Use: ln 2 = 0.7)
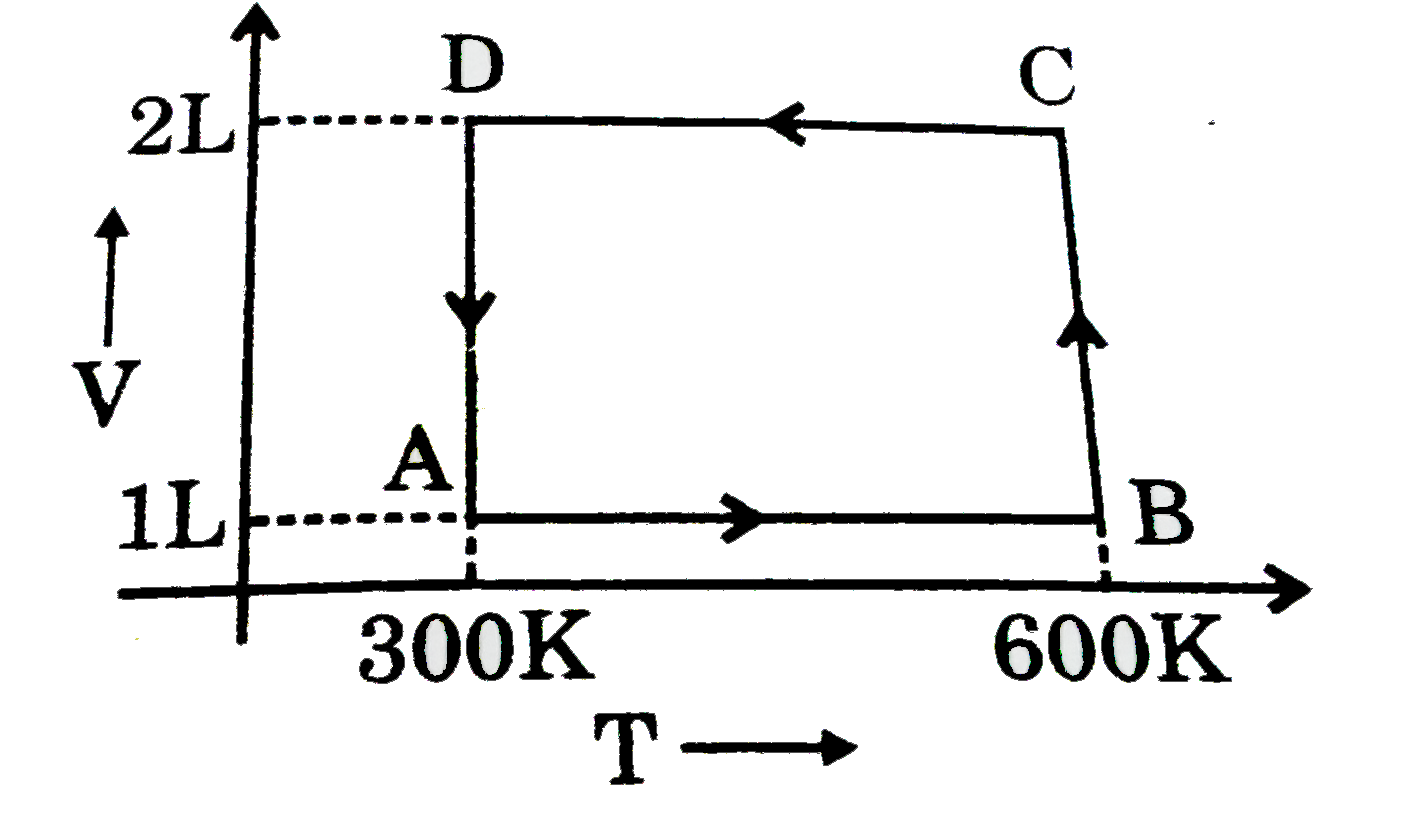
View SolutionCalculate work done for an ideal gas( In 2=0.7)

View SolutionCalculate total number of SO2 molcules in a sample having 32 miligrams of the SO2 gas, 1.4×1020 number of SO2 molcules, 0.8 ml of SO2 gas at 6 atm & 300 K. [Given NA=6×1023,R=0.08 atm lit/mol K]
[Express your answer in terms of multiple of 1020 and then round off to nearest integer for e.g. if your answer is 6.2×1020 fill 6 in OMR after rounding off.]
View SolutionCalculate magnitude of ΔH in calorie for 1 mol of an ideal gas undergoing adiabatic reversible process from 8 atm, 300 K to 2 atm. (Given : γ=2,R=2Cal/K/mol)
View Solution

